Synaptic activity reduces intraneuronal Abeta, promotes APP transport to synapses, and protects against Abeta-related synaptic alterations
- PMID: 19657023
- PMCID: PMC2763626
- DOI: 10.1523/JNEUROSCI.2292-09.2009
Synaptic activity reduces intraneuronal Abeta, promotes APP transport to synapses, and protects against Abeta-related synaptic alterations
Abstract
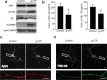
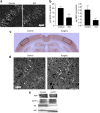
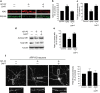
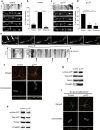
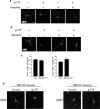
A central question in Alzheimer's disease research is what role synaptic activity plays in the disease process. Synaptic activity has been shown to induce beta-amyloid peptide release into the extracellular space, and extracellular beta-amyloid has been shown to be toxic to synapses. We now provide evidence that the well established synaptotoxicity of extracellular beta-amyloid requires gamma-secretase processing of amyloid precursor protein. Recent evidence supports an important role for intraneuronal beta-amyloid in the pathogenesis of Alzheimer's disease. We show that synaptic activity reduces intraneuronal beta-amyloid and protects against beta-amyloid-related synaptic alterations. We demonstrate that synaptic activity promotes the transport of the amyloid precursor protein to synapses using live cell imaging, and that the protease neprilysin is involved in reduction of intraneuronal beta-amyloid with synaptic activity.
Figures
Similar articles
-
Impaired β-amyloid secretion in Alzheimer's disease pathogenesis.J Neurosci. 2011 Oct 26;31(43):15384-90. doi: 10.1523/JNEUROSCI.2986-11.2011. J Neurosci. 2011. PMID: 22031884 Free PMC article.
-
Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses.Neurobiol Dis. 2005 Nov;20(2):187-98. doi: 10.1016/j.nbd.2005.02.008. Neurobiol Dis. 2005. PMID: 16242627
-
Effect of Aβ Oligomers on Neuronal APP Triggers a Vicious Cycle Leading to the Propagation of Synaptic Plasticity Alterations to Healthy Neurons.J Neurosci. 2020 Jul 1;40(27):5161-5176. doi: 10.1523/JNEUROSCI.2501-19.2020. Epub 2020 May 22. J Neurosci. 2020. PMID: 32444385 Free PMC article.
-
Consequences of inhibiting amyloid precursor protein processing enzymes on synaptic function and plasticity.Neural Plast. 2012;2012:272374. doi: 10.1155/2012/272374. Epub 2012 Jun 26. Neural Plast. 2012. PMID: 22792491 Free PMC article. Review.
-
Synaptic dysfunction in Alzheimer's disease.Adv Exp Med Biol. 2012;970:573-601. doi: 10.1007/978-3-7091-0932-8_25. Adv Exp Med Biol. 2012. PMID: 22351073 Review.
Cited by
-
Reduction of the expression of the late-onset Alzheimer's disease (AD) risk-factor BIN1 does not affect amyloid pathology in an AD mouse model.J Biol Chem. 2019 Mar 22;294(12):4477-4487. doi: 10.1074/jbc.RA118.006379. Epub 2019 Jan 28. J Biol Chem. 2019. PMID: 30692199 Free PMC article.
-
FE65 proteins regulate NMDA receptor activation-induced amyloid precursor protein processing.J Neurochem. 2011 Oct;119(2):377-88. doi: 10.1111/j.1471-4159.2011.07419.x. Epub 2011 Sep 20. J Neurochem. 2011. PMID: 21824144 Free PMC article.
-
Oral Triphenylmethane Food Dye Analog, Brilliant Blue G, Prevents Neuronal Loss in APPSwDI/NOS2-/- Mouse Model.Curr Alzheimer Res. 2016;13(6):663-77. doi: 10.2174/15672050136661602081424568. Curr Alzheimer Res. 2016. PMID: 26852943 Free PMC article.
-
Axonal amyloid precursor protein and its fragments undergo somatodendritic endocytosis and processing.Mol Biol Cell. 2015 Jan 15;26(2):205-17. doi: 10.1091/mbc.E14-06-1049. Epub 2014 Nov 12. Mol Biol Cell. 2015. PMID: 25392299 Free PMC article.
-
GABA-A receptor modulating steroids in acute and chronic stress; relevance for cognition and dementia?Neurobiol Stress. 2019 Dec 20;12:100206. doi: 10.1016/j.ynstr.2019.100206. eCollection 2020 May. Neurobiol Stress. 2019. PMID: 31921942 Free PMC article.
References
-
- Almeida CG, Tampellini D, Takahashi RH, Greengard P, Lin MT, Snyder EM, Gouras GK. Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiol Dis. 2005;20:187–198. - PubMed
-
- Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. Intraneuronal Abeta causes the onset of early Alzheimer's disease-related cognitive deficits in transgenic mice. Neuron. 2005;45:675–688. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K02 AG028174-03/AG/NIA NIH HHS/United States
- R01 AG027140-01A2/AG/NIA NIH HHS/United States
- R01 NS045677-04/NS/NINDS NIH HHS/United States
- R01 NS045677/NS/NINDS NIH HHS/United States
- AG20729/AG/NIA NIH HHS/United States
- K02 AG028174-02/AG/NIA NIH HHS/United States
- AG028174/AG/NIA NIH HHS/United States
- P01 AG009464/AG/NIA NIH HHS/United States
- R01 AG027140-02/AG/NIA NIH HHS/United States
- K02 AG028174/AG/NIA NIH HHS/United States
- AG09464/AG/NIA NIH HHS/United States
- AG027140/AG/NIA NIH HHS/United States
- R01 AG020729/AG/NIA NIH HHS/United States
- R01 AG027140/AG/NIA NIH HHS/United States
- R01 NS045677-05/NS/NINDS NIH HHS/United States
- K02 AG028174-01A2/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
