Small-molecule multidrug resistance-associated protein 1 inhibitor reversan increases the therapeutic index of chemotherapy in mouse models of neuroblastoma
- PMID: 19654298
- PMCID: PMC2746061
- DOI: 10.1158/0008-5472.CAN-09-1075
Small-molecule multidrug resistance-associated protein 1 inhibitor reversan increases the therapeutic index of chemotherapy in mouse models of neuroblastoma
Abstract
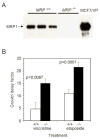
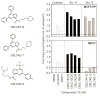
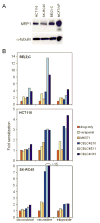
The multidrug resistance-associated protein 1 (MRP1) has been closely linked to poor treatment response in several cancers, most notably neuroblastoma. Homozygous deletion of the MRP1 gene in primary murine neuroblastoma tumors resulted in increased sensitivity to MRP1 substrate drugs (vincristine, etoposide, and doxorubicin) compared with tumors containing both copies of wild-type MRP1, indicating that MRP1 plays a significant role in the drug resistance in this tumor type and defining this multidrug transporter as a target for pharmacologic suppression. A cell-based readout system was created to functionally determine intracellular accumulation of MRP1 substrates using a p53-responsive reporter as an indicator of drug-induced DNA damage. Screening of small-molecule libraries in this readout system revealed pyrazolopyrimidines as a prominent structural class of potent MRP1 inhibitors. Reversan, the lead compound of this class, increased the efficacy of both vincristine and etoposide in murine models of neuroblastoma (syngeneic and human xenografts). As opposed to the majority of inhibitors of multidrug transporters, Reversan was not toxic by itself nor did it increase the toxicity of chemotherapeutic drug exposure in mice. Therefore, Reversan represents a new class of nontoxic MRP1 inhibitor, which may be clinically useful for the treatment of neuroblastoma and other MRP1-overexpressing drug-refractory tumors by increasing their sensitivity to conventional chemotherapy.
Figures

Similar articles
-
In vitro, in vivo and ex vivo characterization of ibrutinib: a potent inhibitor of the efflux function of the transporter MRP1.Br J Pharmacol. 2014 Dec;171(24):5845-57. doi: 10.1111/bph.12889. Epub 2014 Nov 24. Br J Pharmacol. 2014. PMID: 25164592 Free PMC article.
-
ABCC multidrug transporters in childhood neuroblastoma: clinical and biological effects independent of cytotoxic drug efflux.J Natl Cancer Inst. 2011 Aug 17;103(16):1236-51. doi: 10.1093/jnci/djr256. Epub 2011 Jul 28. J Natl Cancer Inst. 2011. PMID: 21799180 Free PMC article.
-
In vitro and in vivo downregulation of MRP1 by antisense oligonucleotides: a potential role in neuroblastoma therapy.Int J Cancer. 2002 Mar 1;98(1):128-33. doi: 10.1002/ijc.10159. Int J Cancer. 2002. PMID: 11857396
-
Role of the MRP1/ABCC1 multidrug transporter protein in cancer.IUBMB Life. 2007 Dec;59(12):752-7. doi: 10.1080/15216540701736285. IUBMB Life. 2007. PMID: 18085475 Review.
-
Small-molecule inhibitors of multidrug resistance-associated protein 1 and related processes: A historic approach and recent advances.Med Res Rev. 2019 Jan;39(1):176-264. doi: 10.1002/med.21510. Epub 2018 May 29. Med Res Rev. 2019. PMID: 29809286 Review.
Cited by
-
Modular tissue-in-a-CUBE platform to model blood-brain barrier (BBB) and brain interaction.Commun Biol. 2024 Feb 28;7(1):177. doi: 10.1038/s42003-024-05857-8. Commun Biol. 2024. PMID: 38418614 Free PMC article.
-
γ-Glutamyltransferase enzyme activity of cancer cells modulates L-γ-glutamyl-p-nitroanilide (GPNA) cytotoxicity.Sci Rep. 2019 Jan 29;9(1):891. doi: 10.1038/s41598-018-37385-x. Sci Rep. 2019. PMID: 30696905 Free PMC article.
-
EFHD2 contributes to non-small cell lung cancer cisplatin resistance by the activation of NOX4-ROS-ABCC1 axis.Redox Biol. 2020 Jul;34:101571. doi: 10.1016/j.redox.2020.101571. Epub 2020 May 16. Redox Biol. 2020. PMID: 32446175 Free PMC article.
-
Inhibition or knockdown of ABC transporters enhances susceptibility of adult and juvenile schistosomes to Praziquantel.PLoS Negl Trop Dis. 2014 Oct 16;8(10):e3265. doi: 10.1371/journal.pntd.0003265. eCollection 2014 Oct. PLoS Negl Trop Dis. 2014. PMID: 25330312 Free PMC article.
-
Lipid-derived electrophiles inhibit the function of membrane channels during ferroptosis.Proc Natl Acad Sci U S A. 2024 May 21;121(21):e2317616121. doi: 10.1073/pnas.2317616121. Epub 2024 May 14. Proc Natl Acad Sci U S A. 2024. PMID: 38743627 Free PMC article.
References
-
- Borst P, Evers R, Kool M, Wijnholds J. A family of drug transporters: the multidrug resistance-associated proteins. Journal of the National Cancer Institute. 2000;92:1295–302. - PubMed
-
- Hipfner R, Deeley R, Cole S. Structural, mechanistic and clinical aspects of MRP1. Biochimica et Biophysica Acta. 1999;1461:359–76. - PubMed
-
- Kuss B, Corbo M, Lau W, Fennell D, Dean N, Cotter F. In vitro and in vivo downregulation of MRP1 by antisense oligonucleotides: a potential role in neuroblastoma therapy. International Journal of Cancer. 2002;98:128–33. - PubMed
-
- Haber M, Bordow S, Gilbert J, et al. Altered expression of the MYCN oncogene modulates MRP expression and response to cytotoxic drugs in neuroblastoma cells. Oncogene. 1999;18:2777–82. - PubMed
-
- Norris MD, Madafiglio J, Gilbert J, Marshall GM, Haber M. Reversal of multidrug resistance-associated protein-mediated drug resistance in cultured human neuroblastoma cells by the quinolone antibiotic difloxacin. Med Pediatr Oncol. 2001;36:177–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

