Tau fragmentation, aggregation and clearance: the dual role of lysosomal processing
- PMID: 19654187
- PMCID: PMC2758146
- DOI: 10.1093/hmg/ddp367
Tau fragmentation, aggregation and clearance: the dual role of lysosomal processing
Abstract
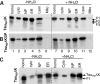
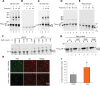
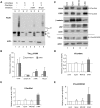
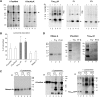
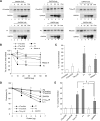
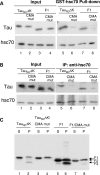
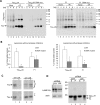
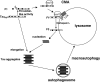
Aggregation and cleavage are two hallmarks of Tau pathology in Alzheimer disease (AD), and abnormal fragmentation of Tau is thought to contribute to the nucleation of Tau paired helical filaments. Clearance of the abnormally modified protein could occur by the ubiquitin-proteasome and autophagy-lysosomal pathways, the two major routes for protein degradation in cells. There is a debate on which of these pathways contributes to clearance of Tau protein and of the abnormal Tau aggregates formed in AD. Here, we demonstrate in an inducible neuronal cell model of tauopathy that the autophagy-lysosomal system contributes to both Tau fragmentation into pro-aggregating forms and to clearance of Tau aggregates. Inhibition of macroautophagy enhances Tau aggregation and cytotoxicity. The Tau repeat domain can be cleaved near the N terminus by a cytosolic protease to generate the fragment F1. Additional cleavage near the C terminus by the lysosomal protease cathepsin L is required to generate Tau fragments F2 and F3 that are highly amyloidogenic and capable of seeding the aggregation of Tau. We identify in this work that components of a selective form of autophagy, chaperone-mediated autophagy, are involved in the delivery of cytosolic Tau to lysosomes for this limited cleavage. However, F1 does not fully enter the lysosome but remains associated with the lysosomal membrane. Inefficient translocation of the Tau fragments across the lysosomal membrane seems to promote formation of Tau oligomers at the surface of these organelles which may act as precursors of aggregation and interfere with lysosomal functioning.
Figures

Similar articles
-
Decrease of neuronal FKBP4/FKBP52 modulates perinuclear lysosomal positioning and MAPT/Tau behavior during MAPT/Tau-induced proteotoxic stress.Autophagy. 2021 Nov;17(11):3491-3510. doi: 10.1080/15548627.2021.1875611. Epub 2021 Jan 25. Autophagy. 2021. PMID: 33459145 Free PMC article.
-
Mask mitigates MAPT- and FUS-induced degeneration by enhancing autophagy through lysosomal acidification.Autophagy. 2017;13(11):1924-1938. doi: 10.1080/15548627.2017.1362524. Epub 2017 Sep 18. Autophagy. 2017. PMID: 28806139 Free PMC article.
-
Generation of tau aggregates and clearance by autophagy in an inducible cell model of tauopathy.Neurodegener Dis. 2010;7(1-3):103-7. doi: 10.1159/000285516. Epub 2010 Feb 18. Neurodegener Dis. 2010. PMID: 20173337
-
Synergy and antagonism of macroautophagy and chaperone-mediated autophagy in a cell model of pathological tau aggregation.Autophagy. 2010 Jan;6(1):182-3. doi: 10.4161/auto.6.1.10815. Epub 2010 Jan 1. Autophagy. 2010. PMID: 20023429 Review.
-
Autophagic Pathways to Clear the Tau Aggregates in Alzheimer's Disease.Cell Mol Neurobiol. 2021 Aug;41(6):1175-1181. doi: 10.1007/s10571-020-00897-0. Epub 2020 Jun 11. Cell Mol Neurobiol. 2021. PMID: 32529542 Free PMC article. Review.
Cited by
-
Endolysosome involvement in LDL cholesterol-induced Alzheimer's disease-like pathology in primary cultured neurons.Life Sci. 2012 Dec 10;91(23-24):1159-68. doi: 10.1016/j.lfs.2012.04.039. Epub 2012 May 11. Life Sci. 2012. PMID: 22580286 Free PMC article.
-
Roles of AMP-activated protein kinase in Alzheimer's disease.Neuromolecular Med. 2012 Mar;14(1):1-14. doi: 10.1007/s12017-012-8173-2. Epub 2012 Feb 26. Neuromolecular Med. 2012. PMID: 22367557 Review.
-
TFEB Overexpression in the P301S Model of Tauopathy Mitigates Increased PHF1 Levels and Lipofuscin Puncta and Rescues Memory Deficits.eNeuro. 2016 May 23;3(2):ENEURO.0042-16.2016. doi: 10.1523/ENEURO.0042-16.2016. eCollection 2016 Mar-Apr. eNeuro. 2016. PMID: 27257626 Free PMC article.
-
Pseudophosphorylation of tau at S422 enhances SDS-stable dimer formation and impairs both anterograde and retrograde fast axonal transport.Exp Neurol. 2016 Sep;283(Pt A):318-29. doi: 10.1016/j.expneurol.2016.06.030. Epub 2016 Jun 30. Exp Neurol. 2016. PMID: 27373205 Free PMC article.
-
FKBP52 in Neuronal Signaling and Neurodegenerative Diseases: A Microtubule Story.Int J Mol Sci. 2022 Feb 3;23(3):1738. doi: 10.3390/ijms23031738. Int J Mol Sci. 2022. PMID: 35163662 Free PMC article. Review.
References
-
- Lee V.M., Goedert M., Trojanowski J.Q. Neurodegenerative tauopathies. Annu. Rev. Neurosci. 2001;24:1121–1159. - PubMed
-
- Garcia M.L., Cleveland D.W. Going new places using an old MAP: tau, microtubules and human neurodegenerative disease. Curr. Opin. Cell Biol. 2001;13:41–48. - PubMed
-
- Johnson G.V., Seubert P., Cox T.M., Motter R., Brown J.P., Galasko D. The tau protein in human cerebrospinal fluid in Alzheimer's disease consists of proteolytically derived fragments. J. Neurochem. 1997;68:430–433. - PubMed
-
- Portelius E., Hansson S.F., Tran A.J., Zetterberg H., Grognet P., Vanmechelen E., Hoglund K., Brinkmalm G., Westman-Brinkmalm A., Nordhoff E., et al. Characterization of tau in cerebrospinal fluid using mass spectrometry. J. Proteome Res. 2008;7:2114–2120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

