Interleukin-6 maintains bone marrow-derived mesenchymal stem cell stemness by an ERK1/2-dependent mechanism
- PMID: 19650110
- PMCID: PMC2774119
- DOI: 10.1002/jcb.22289
Interleukin-6 maintains bone marrow-derived mesenchymal stem cell stemness by an ERK1/2-dependent mechanism
Abstract
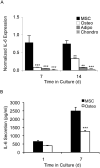
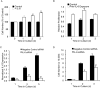
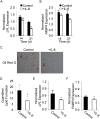
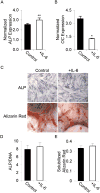
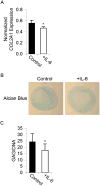
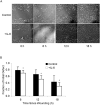
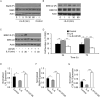
Adult human mesenchymal stem cells (MSCs) hold promise for an increasing list of therapeutic uses due to their ease of isolation, expansion, and multi-lineage differentiation potential. To maximize the clinical potential of MSCs, the underlying mechanisms by which MSC functionality is controlled must be understood. We have taken a deconstructive approach to understand the individual components in vitro, namely the role of candidate "stemness" genes. Our recent microarray gene expression profiling data suggest that interleukin-6 (IL-6) may contribute to the maintenance of MSCs in their undifferentiated state. In this study, we showed that IL-6 gene expression is significantly higher in undifferentiated MSCs as compared to their chondrogenic, osteogenic, and adipogenic derivatives. Moreover, we found that MSCs secrete copious amounts of IL-6 protein, which decreases dramatically during osteogenic differentiation. We further evaluated the role of IL-6 for maintenance of MSC "stemness," using a series of functional assays. The data showed that IL-6 is both necessary and sufficient for enhanced MSC proliferation, protects MSCs from apoptosis, inhibits adipogenic and chondrogenic differentiation of MSCs, and increases the rate of in vitro wound healing of MSCs. We further identified ERK1/2 activation as the key pathway through which IL-6 regulates both MSC proliferation and inhibition of differentiation. Taken together, these findings show for the first time that IL-6 maintains the proliferative and undifferentiated state of bone marrow-derived MSCs, an important parameter for the optimization of both in vitro and in vivo manipulation of MSCs.
(c) 2009 Wiley-Liss, Inc.
Figures

Similar articles
-
Interleukin-6/interleukin-6 receptor complex promotes osteogenic differentiation of bone marrow-derived mesenchymal stem cells.Stem Cell Res Ther. 2018 Jan 22;9(1):13. doi: 10.1186/s13287-017-0766-0. Stem Cell Res Ther. 2018. PMID: 29357923 Free PMC article.
-
Tcf12, A Member of Basic Helix-Loop-Helix Transcription Factors, Mediates Bone Marrow Mesenchymal Stem Cell Osteogenic Differentiation In Vitro and In Vivo.Stem Cells. 2017 Feb;35(2):386-397. doi: 10.1002/stem.2491. Epub 2016 Sep 27. Stem Cells. 2017. PMID: 27574032
-
In vitro effects of RU486 on proliferation and differentiation capabilities of human bone marrow mesenchymal stromal cells.Steroids. 2012 Jan;77(1-2):132-7. doi: 10.1016/j.steroids.2011.10.017. Epub 2011 Nov 7. Steroids. 2012. PMID: 22093480 Free PMC article.
-
Towards Physiologic Culture Approaches to Improve Standard Cultivation of Mesenchymal Stem Cells.Cells. 2021 Apr 13;10(4):886. doi: 10.3390/cells10040886. Cells. 2021. PMID: 33924517 Free PMC article. Review.
-
Regulation Mechanisms and Maintenance Strategies of Stemness in Mesenchymal Stem Cells.Stem Cell Rev Rep. 2024 Feb;20(2):455-483. doi: 10.1007/s12015-023-10658-3. Epub 2023 Nov 27. Stem Cell Rev Rep. 2024. PMID: 38010581 Review.
Cited by
-
Effect of the 3D Artificial Nichoid on the Morphology and Mechanobiological Response of Mesenchymal Stem Cells Cultured In Vitro.Cells. 2020 Aug 11;9(8):1873. doi: 10.3390/cells9081873. Cells. 2020. PMID: 32796521 Free PMC article.
-
Interleukin-17 Family Cytokines in Metabolic Disorders and Cancer.Genes (Basel). 2022 Sep 13;13(9):1643. doi: 10.3390/genes13091643. Genes (Basel). 2022. PMID: 36140808 Free PMC article. Review.
-
Human mesenchymal stem cells derived from umbilical cord and bone marrow exert immunomodulatory effects in different mechanisms.World J Stem Cells. 2020 Sep 26;12(9):1032-1049. doi: 10.4252/wjsc.v12.i9.1032. World J Stem Cells. 2020. PMID: 33033563 Free PMC article.
-
Combination therapies enhance immunoregulatory properties of MIAMI cells.Stem Cell Res Ther. 2019 Dec 18;10(1):395. doi: 10.1186/s13287-019-1515-3. Stem Cell Res Ther. 2019. PMID: 31852519 Free PMC article.
-
Effect of Comedications and Endotoxins on Mesenchymal Stem Cell Secretomes, Migratory and Immunomodulatory Capacity.J Clin Med. 2019 Apr 11;8(4):497. doi: 10.3390/jcm8040497. J Clin Med. 2019. PMID: 30979082 Free PMC article.
References
-
- Akiba H, Nakano H, Nishinaka S, Shindo M, Kobata T, Atsuta M, Morimoto C, Ware CF, Malinin NL, Wallach D, Yagita H, Okumura K. CD27, a member of the tumor necrosis factor receptor superfamily, activates NF-kappaB and stress-activated protein kinase/c-Jun N-terminal kinase via TRAF2, TRAF5, and NF-kappaB-inducing kinase. J Biol Chem. 1998;273:13353–8. - PubMed
-
- Bernad A, Kopf M, Kulbacki R, Weich N, Koehler G, Gutierrez-Ramos JC. Interleukin-6 is required in vivo for the regulation of stem cells and committed progenitors of the hematopoietic system. Immunity. 1994;1:725–31. - PubMed
-
- Burdon T, Smith A, Savatier P. Signalling, cell cycle and pluripotency in embryonic stem cells. Trends Cell Biol. 2002;12:432–8. - PubMed
-
- Camargo CA, Jr, Madden JF, Gao W, Selvan RS, Clavien PA. Interleukin-6 protects liver against warm ischemia/reperfusion injury and promotes hepatocyte proliferation in the rodent. Hepatology. 1997;26:1513–20. - PubMed
-
- Conget PA, Minguell JJ. Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. J Cell Physiol. 1999;181:67–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

