Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner
- PMID: 19648648
- PMCID: PMC2785667
- DOI: 10.1074/jbc.M109.044065
Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner
Abstract
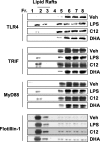
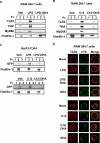
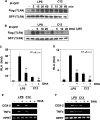
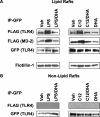
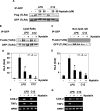
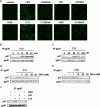
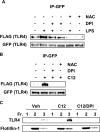
The saturated fatty acids acylated on Lipid A of lipopolysaccharide (LPS) or bacterial lipoproteins play critical roles in ligand recognition and receptor activation for Toll-like Receptor 4 (TLR4) and TLR2. The results from our previous studies demonstrated that saturated and polyunsaturated fatty acids reciprocally modulate the activation of TLR4. However, the underlying mechanism has not been understood. Here, we report for the first time that the saturated fatty acid lauric acid induced dimerization and recruitment of TLR4 into lipid rafts, however, dimerization was not observed in non-lipid raft fractions. Similarly, LPS and lauric acid enhanced the association of TLR4 with MD-2 and downstream adaptor molecules, TRIF and MyD88, into lipid rafts leading to the activation of downstream signaling pathways and target gene expression. However, docosahexaenoic acid (DHA), an n-3 polyunsaturated fatty acid, inhibited LPS- or lauric acid-induced dimerization and recruitment of TLR4 into lipid raft fractions. Together, these results demonstrate that lauric acid and DHA reciprocally modulate TLR4 activation by regulation of the dimerization and recruitment of TLR4 into lipid rafts. In addition, we showed that TLR4 recruitment to lipid rafts and dimerization were coupled events mediated at least in part by NADPH oxidase-dependent reactive oxygen species generation. These results provide a new insight in understanding the mechanism by which fatty acids differentially modulate TLR4-mediated signaling pathway and consequent inflammatory responses which are implicated in the development and progression of many chronic diseases.
Figures
Similar articles
-
Mechanisms for the activation of Toll-like receptor 2/4 by saturated fatty acids and inhibition by docosahexaenoic acid.Eur J Pharmacol. 2016 Aug 15;785:24-35. doi: 10.1016/j.ejphar.2016.04.024. Epub 2016 Apr 13. Eur J Pharmacol. 2016. PMID: 27085899 Free PMC article. Review.
-
Soyasaponin Bb inhibits the recruitment of toll-like receptor 4 (TLR4) into lipid rafts and its signaling pathway by suppressing the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-dependent generation of reactive oxygen species.Mol Nutr Food Res. 2016 Jul;60(7):1532-43. doi: 10.1002/mnfr.201600015. Epub 2016 Apr 21. Mol Nutr Food Res. 2016. PMID: 27005845
-
Reciprocal modulation of Toll-like receptor-4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids.J Biol Chem. 2003 Sep 26;278(39):37041-51. doi: 10.1074/jbc.M305213200. Epub 2003 Jul 15. J Biol Chem. 2003. PMID: 12865424
-
Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1.J Biol Chem. 2004 Apr 23;279(17):16971-9. doi: 10.1074/jbc.M312990200. Epub 2004 Feb 13. J Biol Chem. 2004. PMID: 14966134
-
Saturated fatty acids trigger TLR4-mediated inflammatory response.Atherosclerosis. 2016 Jan;244:211-5. doi: 10.1016/j.atherosclerosis.2015.11.015. Epub 2015 Dec 2. Atherosclerosis. 2016. PMID: 26687466 Review.
Cited by
-
Omega-3 fatty acid intake suppresses induction of diverse autoantibody repertoire by crystalline silica in lupus-prone mice.Autoimmunity. 2020 Nov;53(7):415-433. doi: 10.1080/08916934.2020.1801651. Epub 2020 Sep 9. Autoimmunity. 2020. PMID: 32903098 Free PMC article.
-
Lipids and Suicide Risk.Curr Top Behav Neurosci. 2020;46:155-177. doi: 10.1007/7854_2020_163. Curr Top Behav Neurosci. 2020. PMID: 32840861
-
Athletes Can Benefit from Increased Intake of EPA and DHA-Evaluating the Evidence.Nutrients. 2023 Nov 26;15(23):4925. doi: 10.3390/nu15234925. Nutrients. 2023. PMID: 38068783 Free PMC article. Review.
-
Electrophilic nitro-fatty acids inhibit vascular inflammation by disrupting LPS-dependent TLR4 signalling in lipid rafts.Cardiovasc Res. 2013 Apr 1;98(1):116-24. doi: 10.1093/cvr/cvt002. Epub 2013 Jan 17. Cardiovasc Res. 2013. PMID: 23334216 Free PMC article.
-
Mechanisms for the activation of Toll-like receptor 2/4 by saturated fatty acids and inhibition by docosahexaenoic acid.Eur J Pharmacol. 2016 Aug 15;785:24-35. doi: 10.1016/j.ejphar.2016.04.024. Epub 2016 Apr 13. Eur J Pharmacol. 2016. PMID: 27085899 Free PMC article. Review.
References
-
- Uematsu S., Akira S. (2006) J. Mol. Med. 84, 712–725 - PubMed
-
- Kawai T., Akira S. (2007) Trends Mol. Med. 13, 460–469 - PubMed
-
- Poltorak A., He X., Smirnova I., Liu M. Y., Van Huffel C., Du X., Birdwell D., Alejos E., Silva M., Galanos C., Freudenberg M., Ricciardi-Castagnoli P., Layton B., Beutler B. (1998) Science 282, 2085–2088 - PubMed
-
- Saitoh S., Miyake K. (2006) Chem. Rec. 6, 311–319 - PubMed
-
- Munford R. S., Hall C. L. (1986) Science 234, 203–205 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- C06 RR12088-01/RR/NCRR NIH HHS/United States
- R01 CA075613/CA/NCI NIH HHS/United States
- C06 RR017348/RR/NCRR NIH HHS/United States
- HL079904/HL/NHLBI NIH HHS/United States
- DK064007/DK/NIDDK NIH HHS/United States
- C06 RR012088/RR/NCRR NIH HHS/United States
- DK41868/DK/NIDDK NIH HHS/United States
- R01 DK064007/DK/NIDDK NIH HHS/United States
- R01 HL055330/HL/NHLBI NIH HHS/United States
- HL6234/HL/NHLBI NIH HHS/United States
- R01 HL079904/HL/NHLBI NIH HHS/United States
- HL55330/HL/NHLBI NIH HHS/United States
- CA75613/CA/NCI NIH HHS/United States
- C06 RR17348-01/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

