Functional role of canonical transient receptor potential 1 and canonical transient receptor potential 3 in normal and asthmatic airway smooth muscle cells
- PMID: 19648473
- PMCID: PMC2911567
- DOI: 10.1165/rcmb.2009-0091OC
Functional role of canonical transient receptor potential 1 and canonical transient receptor potential 3 in normal and asthmatic airway smooth muscle cells
Abstract
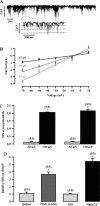
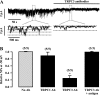
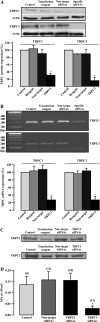
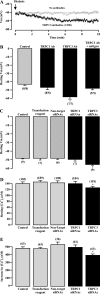
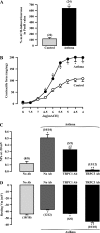
Canonical transient receptor potential (TRPC)-encoded nonselective cation channels (NSCCs) are crucial for many cellular responses in a variety of cells; however, their molecular expression and functional roles in airway smooth muscle cells (ASMCs) remain obscure. The objective of this study was to determine whether TRPC1 and TRPC3 molecules could be important molecular constituents of native NSCCs controlling the resting membrane potential (Vm) and [Ca(2+)](i) in freshly isolated normal and ovalbumin (OVA)-sensitized/-challenged mouse ASMCs. Western blotting, RT-PCR, single-channel recording, whole-cell current-clamp recording, and a fluorescence imaging system were used to determine TRPC expression, NSCC activity, resting Vm, and resting [Ca(2+)](i). Specific individual TRPC antibodies and siRNAs were applied to test their functional roles. TRPC1 and TRPC3 proteins and mRNAs were expressed in freshly isolated ASM tissues. TRPC3 antibodies blocked the activity of NSCCs and hyperpolarized the resting Vm in ASMCs, whereas TRPC1 antibodies had no effect. TRPC3, but not TRPC1 gene silencing, largely diminished NSCC activity, hyperpolarized the resting Vm, lowered the resting [Ca(2+)](i), and inhibited methacholine-induced increase in [Ca(2+)](i). In OVA-sensitized/-challenged ASMCs, NSCC activity was greatly augmented, resting Vm was depolarized, and TRPC3 protein expression was increased. TRPC1 and TRPC3 antibodies blocked the increased activity of NSCCs and membrane depolarization in OVA-sensitized/-challenged cells. TRPC3 is an important molecular component of native NSCCs contributing to the resting Vm and [Ca(2+)](i) in normal ASMCs, as well as membrane depolarization and hyperresponsiveness in OVA-sensitized/-challenged cells, whereas TRPC1-encoded NSCCs are only activated in OVA-sensitized/-challenged airway myocytes.
Figures


Similar articles
-
Molecular expression and functional role of canonical transient receptor potential channels in airway smooth muscle cells.Adv Exp Med Biol. 2011;704:731-47. doi: 10.1007/978-94-007-0265-3_38. Adv Exp Med Biol. 2011. PMID: 21290324 Free PMC article. Review.
-
The effects of transient receptor potential channel (TRPC) on airway smooth muscle cell isolated from asthma model mice.J Cell Biochem. 2018 Jul;119(7):6033-6044. doi: 10.1002/jcb.26801. Epub 2018 Mar 25. J Cell Biochem. 2018. PMID: 29574924
-
TRPC3-mediated Ca(2+) entry contributes to mouse airway smooth muscle cell proliferation induced by lipopolysaccharide.Cell Calcium. 2016 Oct;60(4):273-81. doi: 10.1016/j.ceca.2016.06.005. Epub 2016 Jun 23. Cell Calcium. 2016. PMID: 27377672
-
TRPC3 overexpression and intervention in airway smooth muscle of ovalbumin-induced hyperresponsiveness and remodeling.Cell Biol Int. 2018 Aug;42(8):1021-1029. doi: 10.1002/cbin.10970. Epub 2018 Apr 26. Cell Biol Int. 2018. PMID: 29624776
-
Canonical Transient Potential Receptor-3 Channels in Normal and Diseased Airway Smooth Muscle Cells.Adv Exp Med Biol. 2020;1131:471-487. doi: 10.1007/978-3-030-12457-1_18. Adv Exp Med Biol. 2020. PMID: 31646521 Review.
Cited by
-
Maintenance of intracellular Ca2+ basal concentration in airway smooth muscle (Review).Int J Mol Med. 2018 Dec;42(6):2998-3008. doi: 10.3892/ijmm.2018.3910. Epub 2018 Oct 2. Int J Mol Med. 2018. PMID: 30280184 Free PMC article. Review.
-
Calcium released by osteoclastic resorption stimulates autocrine/paracrine activities in local osteogenic cells to promote coupled bone formation.Am J Physiol Cell Physiol. 2022 May 1;322(5):C977-C990. doi: 10.1152/ajpcell.00413.2021. Epub 2022 Apr 6. Am J Physiol Cell Physiol. 2022. PMID: 35385325 Free PMC article.
-
Airway smooth muscle in airway reactivity and remodeling: what have we learned?Am J Physiol Lung Cell Mol Physiol. 2013 Dec;305(12):L912-33. doi: 10.1152/ajplung.00259.2013. Epub 2013 Oct 18. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 24142517 Free PMC article. Review.
-
Molecular expression and functional role of canonical transient receptor potential channels in airway smooth muscle cells.Adv Exp Med Biol. 2011;704:731-47. doi: 10.1007/978-94-007-0265-3_38. Adv Exp Med Biol. 2011. PMID: 21290324 Free PMC article. Review.
-
Cellular Na+ handling mechanisms involved in airway smooth muscle contraction (Review).Int J Mol Med. 2017 Jul;40(1):3-9. doi: 10.3892/ijmm.2017.2993. Epub 2017 May 17. Int J Mol Med. 2017. PMID: 28534960 Free PMC article. Review.
References
-
- Montell C, Birnbaumer L, Flockerzi V, Bindels RJ, Bruford EA, Caterina MJ, Clapham DE, Harteneck C, Heller S, Julius D, et al. A unified nomenclature for the superfamily of TRP cation channels. Mol Cell 2002;9:229–231. - PubMed
-
- Clapham DE, Montell C, Schultz G, Julius D. International Union of Pharmacology. XLIII. Compendium of voltage-gated ion channels: transient receptor potential channels. Pharmacol Rev 2003;55:591–596. - PubMed
-
- Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev 2007;87:165–217. - PubMed
-
- Ong HL, Brereton HM, Harland ML, Barritt GJ. Evidence for the expression of transient receptor potential proteins in guinea pig airway smooth muscle cells. Respirology 2003;8:23–32. - PubMed
-
- Corteling RL, Li S, Giddings J, Westwick J, Poll C, Hall IP. Expression of transient receptor potential C6 and related transient receptor potential family members in human airway smooth muscle and lung tissue. Am J Respir Cell Mol Biol 2004;30:145–154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

