Phosphorylation of histone H3 by protein kinase C signaling plays a critical role in the regulation of the developmentally important TBX2 gene
- PMID: 19633291
- PMCID: PMC2785324
- DOI: 10.1074/jbc.M109.021360
Phosphorylation of histone H3 by protein kinase C signaling plays a critical role in the regulation of the developmentally important TBX2 gene
Abstract
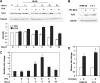
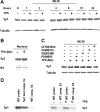
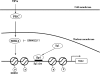
The mechanism(s) regulating the expression of the TBX2 gene, a key regulator of development, is poorly understood and thus limits an understanding of its function(s). Here we demonstrate that 12-O-tetradecanoylphorbol-13-acetate (TPA) induces TBX2 expression in normal human fibroblasts in a protein kinase C (PKC)-dependent and MAPK-independent manner. Our data further reveal that TPA activates transcription of TBX2 through activating MSK1, which leads to an increase in phosphorylated histone H3 and the recruitment of Sp1 to the TBX2 gene. In addition, TPA was shown to activate MSK1 in a PKC-dependent and MAPK-independent manner. This study is the first to provide evidence that phosphorylation of histone H3 leads to the transcriptional activation of the TBX2 gene and to link MSK1 to PKC.
Figures



Similar articles
-
The nucleosomal response associated with immediate-early gene induction is mediated via alternative MAP kinase cascades: MSK1 as a potential histone H3/HMG-14 kinase.EMBO J. 1999 Sep 1;18(17):4779-93. doi: 10.1093/emboj/18.17.4779. EMBO J. 1999. PMID: 10469656 Free PMC article.
-
Interferon-gamma stimulates the expression of the inducible cAMP early repressor in macrophages through the activation of casein kinase 2. A potentially novel pathway for interferon-gamma-mediated inhibition of gene transcription.J Biol Chem. 2003 May 16;278(20):17741-51. doi: 10.1074/jbc.M301602200. Epub 2003 Feb 27. J Biol Chem. 2003. PMID: 12609974
-
Chromatin modification of the trefoil factor 1 gene in human breast cancer cells by the Ras/mitogen-activated protein kinase pathway.Cancer Res. 2006 May 1;66(9):4610-6. doi: 10.1158/0008-5472.CAN-05-4251. Cancer Res. 2006. PMID: 16651411
-
Ser-10 phosphorylation of histone H3 and immediate early gene expression in oncogene-transformed mouse fibroblasts.Cancer Res. 2002 Jan 1;62(1):75-8. Cancer Res. 2002. PMID: 11782362
-
Effect of contraction on mitogen-activated protein kinase signal transduction in skeletal muscle. Involvement Of the mitogen- and stress-activated protein kinase 1.J Biol Chem. 2000 Jan 14;275(2):1457-62. doi: 10.1074/jbc.275.2.1457. J Biol Chem. 2000. PMID: 10625698
Cited by
-
Cytomegalovirus Latency and Reactivation: An Intricate Interplay With the Host Immune Response.Front Cell Infect Microbiol. 2020 Mar 31;10:130. doi: 10.3389/fcimb.2020.00130. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32296651 Free PMC article. Review.
-
Genetic analysis of the TBX2 gene promoter in indirect inguinal hernia.Hernia. 2014 Aug;18(4):513-7. doi: 10.1007/s10029-013-1199-z. Epub 2013 Dec 6. Hernia. 2014. PMID: 24309999
-
Role of calcium-independent phospholipase A2beta in high glucose-induced activation of RhoA, Rho kinase, and CPI-17 in cultured vascular smooth muscle cells and vascular smooth muscle hypercontractility in diabetic animals.J Biol Chem. 2010 Mar 19;285(12):8628-38. doi: 10.1074/jbc.M109.057711. Epub 2010 Jan 19. J Biol Chem. 2010. PMID: 20086008 Free PMC article.
-
Emerging role of protein kinase C in energy homeostasis: A brief overview.World J Diabetes. 2014 Jun 15;5(3):385-92. doi: 10.4239/wjd.v5.i3.385. World J Diabetes. 2014. PMID: 24936260 Free PMC article. Review.
-
Design, Synthesis and Biological Evaluation of Arylpyridin-2-yl Guanidine Derivatives and Cyclic Mimetics as Novel MSK1 Inhibitors. An Application in an Asthma Model.Molecules. 2021 Jan 13;26(2):391. doi: 10.3390/molecules26020391. Molecules. 2021. PMID: 33450992 Free PMC article.
References
-
- Bollag R. J., Siegfried Z., Cebra-Thomas J. A., Garvey N., Davison E. M., Silver L. M. (1994) Nat. Genet. 7, 383–389 - PubMed
-
- Campbell C., Goodrich K., Casey G., Beatty B. (1995) Genomics 28, 255–260 - PubMed
-
- Law D. J., Gebuhr T., Garvey N., Agulnik S. I., Silver L. M. (1995) Mamm. Genome 6, 793–797 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

