Interaction of HCMV UL84 with C/EBPalpha transcription factor binding sites within oriLyt is essential for lytic DNA replication
- PMID: 19631360
- PMCID: PMC2740927
- DOI: 10.1016/j.virol.2009.06.035
Interaction of HCMV UL84 with C/EBPalpha transcription factor binding sites within oriLyt is essential for lytic DNA replication
Abstract
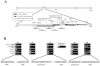
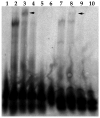
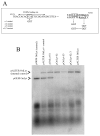
Human cytomegalovirus (HCMV) lytic DNA replication is initiated at the cis-acting oriLyt region and requires six core replication proteins along with UL84 and IE2. Although UL84 is thought to be the replication initiator protein, little is known about its interaction with oriLyt. We have now performed chromatin immunoprecipitation assays (ChIP) using antibodies specific to UL84, IE2, UL44, CCAAT/enhancer binding protein (C/EBPalpha) and PCR primers that span the entire oriLyt region to reveal an evaluation of specific protein binding across oriLyt. UL84 interacted with several regions of oriLyt that contain C/EBPalpha transcription factor binding sites. Mutation of either of one of C/EBPalpha (92,526 or 92,535) sites inactivated oriLyt and resulted in the loss of binding of UL84. These data reveal the regions of interaction within oriLyt for several key replication proteins and show that the interaction between UL84 and C/EBPalpha sites within oriLyt is essential for lytic DNA replication.
Figures


Similar articles
-
Human cytomegalovirus UL84 interacts with an RNA stem-loop sequence found within the RNA/DNA hybrid region of oriLyt.J Virol. 2007 Jul;81(13):7077-85. doi: 10.1128/JVI.00058-07. Epub 2007 Apr 25. J Virol. 2007. PMID: 17459920 Free PMC article.
-
Characteristics of Immediate-Early 2 (IE2) and UL84 Proteins in UL84-Independent Strains of Human Cytomegalovirus (HCMV).Microbiol Spectr. 2021 Oct 31;9(2):e0053921. doi: 10.1128/Spectrum.00539-21. Epub 2021 Sep 22. Microbiol Spectr. 2021. PMID: 34550009 Free PMC article.
-
Human cytomegalovirus DNA replication requires transcriptional activation via an IE2- and UL84-responsive bidirectional promoter element within oriLyt.J Virol. 2004 Nov;78(21):11664-77. doi: 10.1128/JVI.78.21.11664-11677.2004. J Virol. 2004. PMID: 15479808 Free PMC article.
-
Human cytomegalovirus UL84 oligomerization and heterodimerization domains act as transdominant inhibitors of oriLyt-dependent DNA replication: evidence that IE2-UL84 and UL84-UL84 interactions are required for lytic DNA replication.J Virol. 2004 Sep;78(17):9203-14. doi: 10.1128/JVI.78.17.9203-9214.2004. J Virol. 2004. PMID: 15308715 Free PMC article.
-
Nuts and bolts of human cytomegalovirus lytic DNA replication.Curr Top Microbiol Immunol. 2008;325:153-66. doi: 10.1007/978-3-540-77349-8_9. Curr Top Microbiol Immunol. 2008. PMID: 18637505 Review.
Cited by
-
Initiation of lytic DNA replication in Epstein-Barr virus: search for a common family mechanism.Future Virol. 2010 Jan;5(1):65-83. doi: 10.2217/fvl.09.69. Future Virol. 2010. PMID: 22468146 Free PMC article.
-
Depletion of cellular pre-replication complex factors results in increased human cytomegalovirus DNA replication.PLoS One. 2012;7(5):e36057. doi: 10.1371/journal.pone.0036057. Epub 2012 May 7. PLoS One. 2012. PMID: 22586460 Free PMC article.
-
Human cytomegalovirus long noncoding RNA4.9 regulates viral DNA replication.PLoS Pathog. 2020 Apr 15;16(4):e1008390. doi: 10.1371/journal.ppat.1008390. eCollection 2020 Apr. PLoS Pathog. 2020. PMID: 32294138 Free PMC article.
-
pUL34 binding near the human cytomegalovirus origin of lytic replication enhances DNA replication and viral growth.Virology. 2018 May;518:414-422. doi: 10.1016/j.virol.2018.03.017. Epub 2018 Apr 5. Virology. 2018. PMID: 29626748 Free PMC article.
-
Functional annotation of human cytomegalovirus gene products: an update.Front Microbiol. 2014 May 19;5:218. doi: 10.3389/fmicb.2014.00218. eCollection 2014. Front Microbiol. 2014. PMID: 24904534 Free PMC article. Review.
References
-
- AuCoin DP, Colletti KS, Cei SA, Papouskova I, Tarrant M, Pari GS. Amplification of the Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 lytic origin of DNA replication is dependent upon a cis-acting AT-rich region and an ORF50 response element and the trans-acting factors ORF50 (K-Rta) and K8 (K-bZIP) Virology. 2004;318(2):542–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

