Regulation of inflammatory monocyte/macrophage recruitment from the bone marrow during murine cytomegalovirus infection: role for type I interferons in localized induction of CCR2 ligands
- PMID: 19620305
- PMCID: PMC2911023
- DOI: 10.4049/jimmunol.0900205
Regulation of inflammatory monocyte/macrophage recruitment from the bone marrow during murine cytomegalovirus infection: role for type I interferons in localized induction of CCR2 ligands
Abstract
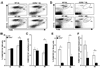
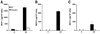
Monocytes/macrophages are critical early innate immune responders during murine CMV (MCMV) infection. It has been established that inflammatory monocyte/macrophages are released from the bone marrow and into the peripheral blood before entry into infected tissue sites. We previously reported a role for IFN-alpha/beta in promotion of CCR2-mediated recruitment of monocyte/macrophages into the liver in response to MCMV infection. However, the mechanisms that support the migration of monocyte/macrophages from the bone marrow and into the peripheral blood under conditions of MCMV infection have not been elucidated. Herein, we demonstrate an accumulation of monocyte/macrophages in the bone marrow of MCMV-infected CCR2-deficient mice, whereas circulating monocyte/macrophages are profoundly diminished. The CCR2 ligands MCP-1, MCP-3, and MCP-5 are detected in bone marrow and in serum from MCMV-infected mice. Furthermore, bone marrow leukocytes from naive mice produce high levels of MCP-1 and MCP-5, and moderate levels of MCP-3, when stimulated with recombinant IFN-alpha in culture. We identify bone marrow F4/80(+) cells as major producers of MCP-1, MCP-3, and MCP-5. Moreover, induction of CCR2 ligands is dependent on IFN-alpha/beta-mediated signals and MCMV infection. Taken together, the results reveal a critical role for inflammatory cytokines in stimulating production of CCR2-binding chemokines from F4/80(+) cells in the bone marrow, and they suggest that local production of chemokines supports monocyte/macrophage egress from the bone marrow into the blood during a virus infection.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures




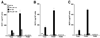
Similar articles
-
Monocyte chemoattractant protein-1 and CCR2 interactions are required for IFN-alpha/beta-induced inflammatory responses and antiviral defense in liver.J Immunol. 2005 Feb 1;174(3):1549-56. doi: 10.4049/jimmunol.174.3.1549. J Immunol. 2005. PMID: 15661915
-
Additive roles for MCP-1 and MCP-3 in CCR2-mediated recruitment of inflammatory monocytes during Listeria monocytogenes infection.J Immunol. 2008 May 15;180(10):6846-53. doi: 10.4049/jimmunol.180.10.6846. J Immunol. 2008. PMID: 18453605 Free PMC article.
-
MyD88 and Type I interferon receptor-mediated chemokine induction and monocyte recruitment during Listeria monocytogenes infection.J Immunol. 2009 Jul 15;183(2):1271-8. doi: 10.4049/jimmunol.0900460. Epub 2009 Jun 24. J Immunol. 2009. PMID: 19553532 Free PMC article.
-
CCR2-deficient mice are protected to sepsis by the disruption of the inflammatory monocytes emigration from the bone marrow.J Leukoc Biol. 2021 Jun;109(6):1063-1070. doi: 10.1002/JLB.4MR0820-049RR. Epub 2020 Oct 5. J Leukoc Biol. 2021. PMID: 33020963 Review.
-
Monocyte subtypes and the CCR2 chemokine receptor in cardiovascular disease.Clin Sci (Lond). 2017 Jun 1;131(12):1215-1224. doi: 10.1042/CS20170009. Clin Sci (Lond). 2017. PMID: 28566450 Review.
Cited by
-
Monocyte-mediated immune defense against murine Listeria monocytogenes infection.Adv Immunol. 2012;113:119-34. doi: 10.1016/B978-0-12-394590-7.00003-8. Adv Immunol. 2012. PMID: 22244581 Free PMC article. Review.
-
Effect of Yiguanjian decoction on cell differentiation and proliferation in CCl₄-treated mice.World J Gastroenterol. 2012 Jul 7;18(25):3235-49. doi: 10.3748/wjg.v18.i25.3235. World J Gastroenterol. 2012. PMID: 22783047 Free PMC article.
-
Modulation of host innate and adaptive immune defenses by cytomegalovirus: timing is everything.J Intern Med. 2010 May;267(5):483-501. doi: 10.1111/j.1365-2796.2010.02220.x. J Intern Med. 2010. PMID: 20433576 Free PMC article. Review.
-
The Mechanism behind Influenza Virus Cytokine Storm.Viruses. 2021 Jul 14;13(7):1362. doi: 10.3390/v13071362. Viruses. 2021. PMID: 34372568 Free PMC article. Review.
-
Interferon response factors 3 and 7 protect against Chikungunya virus hemorrhagic fever and shock.J Virol. 2012 Sep;86(18):9888-98. doi: 10.1128/JVI.00956-12. Epub 2012 Jul 3. J Virol. 2012. PMID: 22761364 Free PMC article.
References
-
- Betts RF, Hanshaw JB. Cytomegalovirus (CMV) in the compromised host(s) Annu. Rev. Med. 1977;28:103–110. - PubMed
-
- Drew WL. Cytomegalovirus infection in patients with AIDS. J. Infect. Dis. 1988;158:449–456. - PubMed
-
- Krmpotic A, Bubic I, Polic B, Lucin P, Jonjic S. Pathogenesis of murine cytomegalovirus infection. Microbes Infect. 2003;5:1263–1277. - PubMed
-
- Scalzo AA, Corbett AJ, Rawlinson WD, Scott GM, Degli-Esposti MA. The interplay between host and viral factors in shaping the outcome of cytomegalovirus infection. Immunol. Cell Biol. 2007;85:46–54. - PubMed
-
- Hokeness-Antonelli KL, Crane MJ, Dragoi AM, Chu WM, Salazar-Mather TP. IFN-α/β-mediated inflammatory responses and antiviral defense in liver is TLR9-independent but MyD88-dependent during murine cytomegalovirus infection. J. Immunol. 2007;179:6176–6183. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

