Claudin-containing exosomes in the peripheral circulation of women with ovarian cancer
- PMID: 19619303
- PMCID: PMC2719664
- DOI: 10.1186/1471-2407-9-244
Claudin-containing exosomes in the peripheral circulation of women with ovarian cancer
Abstract
Background: The absence of highly sensitive and specific serum biomarkers makes mass screening for ovarian cancer impossible. The claudin proteins are frequently overexpressed in ovarian cancers, but their potential as prognostic, diagnostic, or detection markers remains unclear. Here, we have explored the possible use of these proteins as screening biomarkers for ovarian cancer detection.
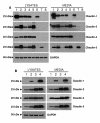
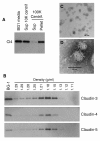
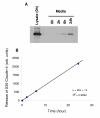
Methods: Claudin protein shedding from cells was examined by immunoblotting of conditioned culture media. The presence of claudins in exosomes released from ovarian cancer cells was demonstrated by sucrose gradient separation and immunogold electron microscopy experiments. Claudin-4-containing exosomes in the plasma of ovarian cancer patients were evaluated in a pilot panel of 63 ovarian cancer patients and 50 healthy volunteers. The CA125 marker was also assessed in these samples and compared with claudin-4 positivity.
Results: We show that full-length claudins can be shed from ovarian cancer cells in culture and found in the media as part of small lipid vesicles known as exosomes. Moreover, 32 of 63 plasma samples from ovarian cancer patients exhibited the presence of claudin-4-containing exosomes. In contrast, only one of 50 samples from individuals without cancer exhibited claudin-4-positive exosomes. In our small panel, at a specificity of 98%, the claudin-4 and CA125 tests had sensitivities of 51% and 71%, respectively. The two tests did not appear to be independent and were strongly correlated.
Conclusion: Our work shows for the first time that claudin-4 can be released from ovarian cancer cells and can be detected in the peripheral circulation of ovarian cancer patients. The development of sensitive assays for the detection of claudin-4 in blood will be crucial in determining whether this approach can be useful, alone or in combination with other screening methods, for the detection of ovarian cancer.
Figures

Similar articles
-
Claudin-7 is frequently overexpressed in ovarian cancer and promotes invasion.PLoS One. 2011;6(7):e22119. doi: 10.1371/journal.pone.0022119. Epub 2011 Jul 15. PLoS One. 2011. PMID: 21789222 Free PMC article.
-
Tight junction proteins claudin-3 and claudin-4 are frequently overexpressed in ovarian cancer but not in ovarian cystadenomas.Clin Cancer Res. 2003 Jul;9(7):2567-75. Clin Cancer Res. 2003. PMID: 12855632
-
Phosphorylation of claudin-3 at threonine 192 by cAMP-dependent protein kinase regulates tight junction barrier function in ovarian cancer cells.J Biol Chem. 2005 Jul 15;280(28):26233-40. doi: 10.1074/jbc.M502003200. Epub 2005 May 19. J Biol Chem. 2005. PMID: 15905176
-
Techniques Associated with Exosome Isolation for Biomarker Development: Liquid Biopsies for Ovarian Cancer Detection.Methods Mol Biol. 2020;2055:181-199. doi: 10.1007/978-1-4939-9773-2_8. Methods Mol Biol. 2020. PMID: 31502152 Review.
-
Early detection of ovarian cancer.Dis Markers. 2007;23(5-6):397-410. doi: 10.1155/2007/309382. Dis Markers. 2007. PMID: 18057523 Free PMC article. Review.
Cited by
-
Exosomes─Nature's Lipid Nanoparticles, a Rising Star in Drug Delivery and Diagnostics.ACS Nano. 2022 Nov 22;16(11):17802-17846. doi: 10.1021/acsnano.2c08774. Epub 2022 Nov 10. ACS Nano. 2022. PMID: 36354238 Free PMC article. Review.
-
An Update on Novel Therapeutic Warfronts of Extracellular Vesicles (EVs) in Cancer Treatment: Where We Are Standing Right Now and Where to Go in the Future.Oxid Med Cell Longev. 2019 Jul 25;2019:9702562. doi: 10.1155/2019/9702562. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31428232 Free PMC article. Review.
-
Current and Emerging Methods for Ovarian Cancer Screening and Diagnostics: A Comprehensive Review.Cancers (Basel). 2022 Jun 11;14(12):2885. doi: 10.3390/cancers14122885. Cancers (Basel). 2022. PMID: 35740550 Free PMC article. Review.
-
Joint analysis of the metabolomics and transcriptomics uncovers the dysregulated network and develops the diagnostic model of high-risk neuroblastoma.Sci Rep. 2023 Oct 9;13(1):16991. doi: 10.1038/s41598-023-43988-w. Sci Rep. 2023. PMID: 37813883 Free PMC article.
-
Claudin-2 promotes colorectal cancer liver metastasis and is a biomarker of the replacement type growth pattern.Commun Biol. 2021 Jun 2;4(1):657. doi: 10.1038/s42003-021-02189-9. Commun Biol. 2021. PMID: 34079064 Free PMC article.
References
-
- Hogdall EV, Christensen L, Kjaer SK, Blaakaer J, Kjaerbye-Thygesen A, Gayther S, Jacobs IJ, Hogdall CK. CA125 expression pattern, prognosis and correlation with serum CA125 in ovarian tumor patients. From The Danish "MALOVA" Ovarian Cancer Study. Gynecol Oncol. 2007;104:508–515. doi: 10.1016/j.ygyno.2006.09.028. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

