Structural basis for recruitment of BRCA2 by PALB2
- PMID: 19609323
- PMCID: PMC2750052
- DOI: 10.1038/embor.2009.126
Structural basis for recruitment of BRCA2 by PALB2
Erratum in
-
Structural basis for recruitment of BRCA2 by PALB2.EMBO Rep. 2017 Jul;18(7):1264. doi: 10.15252/embr.201744508. EMBO Rep. 2017. PMID: 28673926 Free PMC article.
Abstract
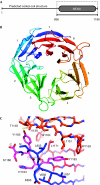
The breast cancer 2, early onset protein (BRCA2) is central to the repair of DNA damage by homologous recombination. BRCA2 recruits the recombinase RAD51 to sites of damage, regulates its assembly into nucleoprotein filaments and thereby promotes homologous recombination. Localization of BRCA2 to nuclear foci requires its association with the partner and localizer of BRCA2 (PALB2), mutations in which are associated with cancer predisposition, as well as subtype N of Fanconi anaemia. We have determined the structure of the PALB2 carboxy-terminal beta-propeller domain in complex with a BRCA2 peptide. The structure shows the molecular determinants of this important protein-protein interaction and explains the effects of both cancer-associated truncating mutants in PALB2 and missense mutations in the amino-terminal region of BRCA2.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Breast cancer-associated missense mutants of the PALB2 WD40 domain, which directly binds RAD51C, RAD51 and BRCA2, disrupt DNA repair.Oncogene. 2014 Oct 2;33(40):4803-12. doi: 10.1038/onc.2013.421. Epub 2013 Oct 21. Oncogene. 2014. PMID: 24141787 Free PMC article.
-
Plasticity of BRCA2 function in homologous recombination: genetic interactions of the PALB2 and DNA binding domains.PLoS Genet. 2011 Dec;7(12):e1002409. doi: 10.1371/journal.pgen.1002409. Epub 2011 Dec 15. PLoS Genet. 2011. PMID: 22194698 Free PMC article.
-
PALB2 functionally connects the breast cancer susceptibility proteins BRCA1 and BRCA2.Mol Cancer Res. 2009 Jul;7(7):1110-8. doi: 10.1158/1541-7786.MCR-09-0123. Epub 2009 Jul 7. Mol Cancer Res. 2009. PMID: 19584259 Free PMC article.
-
PALB2: the hub of a network of tumor suppressors involved in DNA damage responses.Biochim Biophys Acta. 2014 Aug;1846(1):263-75. doi: 10.1016/j.bbcan.2014.06.003. Epub 2014 Jul 3. Biochim Biophys Acta. 2014. PMID: 24998779 Free PMC article. Review.
-
BRCA1-Dependent and Independent Recruitment of PALB2-BRCA2-RAD51 in the DNA Damage Response and Cancer.Cancer Res. 2022 Sep 16;82(18):3191-3197. doi: 10.1158/0008-5472.CAN-22-1535. Cancer Res. 2022. PMID: 35819255 Free PMC article. Review.
Cited by
-
Germline Mutations in Other Homologous Recombination Repair-Related Genes Than BRCA1/2: Predictive or Prognostic Factors?J Pers Med. 2021 Mar 28;11(4):245. doi: 10.3390/jpm11040245. J Pers Med. 2021. PMID: 33800556 Free PMC article.
-
Characterization of a novel germline PALB2 duplication in a hereditary breast and ovarian cancer family.Breast Cancer Res Treat. 2016 Dec;160(3):447-456. doi: 10.1007/s10549-016-4021-7. Epub 2016 Oct 18. Breast Cancer Res Treat. 2016. PMID: 27757719 Free PMC article.
-
Cytotoxic Evaluation, Molecular Docking, and 2D-QSAR Studies of Dihydropyrimidinone Derivatives as Potential Anticancer Agents.J Oncol. 2022 Apr 25;2022:7715689. doi: 10.1155/2022/7715689. eCollection 2022. J Oncol. 2022. PMID: 35509846 Free PMC article.
-
A global functional analysis of missense mutations reveals two major hotspots in the PALB2 tumor suppressor.Nucleic Acids Res. 2019 Nov 18;47(20):10662-10677. doi: 10.1093/nar/gkz780. Nucleic Acids Res. 2019. PMID: 31586400 Free PMC article.
-
Identification of the most common BRCA alterations through analysis of germline mutation databases: Is droplet digital PCR an additional strategy for the assessment of such alterations in breast and ovarian cancer families?Int J Oncol. 2022 May;60(5):58. doi: 10.3892/ijo.2022.5349. Epub 2022 Apr 6. Int J Oncol. 2022. PMID: 35383859 Free PMC article.
References
-
- Daniels MJ, Wang Y, Lee M, Venkitaraman AR (2004) Abnormal cytokinesis in cells deficient in the breast cancer susceptibility protein BRCA2. Science 306: 876–879 - PubMed
-
- Edwards SL, Brough R, Lord CJ, Natrajan R, Vatcheva R, Levine DA, Boyd J, Reis-Filho JS, Ashworth A (2008) Resistance to therapy caused by intragenic deletion in BRCA2. Nature 451: 1111–1115 - PubMed
-
- Esashi F, Galkin VE, Yu X, Egelman EH, West SC (2007) Stabilization of RAD51 nucleoprotein filaments by the C-terminal region of BRCA2. Nat Struct Mol Biol 14: 468–474 - PubMed
-
- Fuks F, Milner J, Kouzarides T (1998) BRCA2 associates with acetyltransferase activity when bound to P/CAF. Oncogene 17: 2531–2534 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

