GnRH pulsatility, the pituitary response and reproductive dysfunction
- PMID: 19609045
- PMCID: PMC4307809
- DOI: 10.1507/endocrj.k09e-185
GnRH pulsatility, the pituitary response and reproductive dysfunction
Abstract
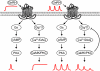
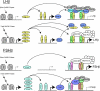
GnRH plays an essential role in neuroendocrine control of reproductive function. In mammals, the pattern of gonadotropin secretion includes both pulse and surge phases, which are regulated independently. The pulsatile release of GnRH and LH plays an important role in the development of sexual function and in the normal regulation of the menstrual cycle. The importance of GnRH pulsatility was established in a series of classic studies. Fertility is impaired when GnRH pulsatility is inhibited by chronic malnutrition, excessive caloric expenditure, or aging. A number of reproductive disorders in women with including hypogonadotropic hypogonadism, hypothlamic amenorrhea, hyperprolactinemia and polycystic ovary syndrome (PCOS) are also associated with disruption of the normal pulsatile GnRH secretion. Despite these findings, the molecular mechanisms of this pulsatile GnRH regulation are not well understood. Here, we review recent studies about GnRH pulsatility, signaling and transcriptional response, and its implications for disease.
Figures
Similar articles
-
Gonadotropin-releasing hormone (GnRH) physiology in men and women.Acta Med Hung. 1986;43(2):201-21. Acta Med Hung. 1986. PMID: 3295743
-
Endocrine response determines the clinical outcome of pulsatile gonadotropin-releasing hormone ovulation induction in different ovulatory disorders.J Clin Endocrinol Metab. 1991 May;72(5):965-72. doi: 10.1210/jcem-72-5-965. J Clin Endocrinol Metab. 1991. PMID: 1902487
-
GnRH and GnRH receptors in the pathophysiology of the human female reproductive system.Hum Reprod Update. 2016 Apr;22(3):358-81. doi: 10.1093/humupd/dmv059. Epub 2015 Dec 29. Hum Reprod Update. 2016. PMID: 26715597 Review.
-
Pulsatile control of reproduction.Lancet. 1984 Aug 18;2(8399):382-3. Lancet. 1984. PMID: 6147459
-
Gonadotropin-releasing hormone pulses: regulators of gonadotropin synthesis and ovulatory cycles.Recent Prog Horm Res. 1991;47:155-87; discussion 188-9. doi: 10.1016/b978-0-12-571147-0.50009-3. Recent Prog Horm Res. 1991. PMID: 1745819 Review.
Cited by
-
Troxerutin protects against DHT-induced polycystic ovary syndrome in rats.J Ovarian Res. 2020 Sep 13;13(1):106. doi: 10.1186/s13048-020-00701-z. J Ovarian Res. 2020. PMID: 32921318 Free PMC article.
-
The Role of GnRH Receptor Autoantibodies in Polycystic Ovary Syndrome.J Endocr Soc. 2020 Jun 18;4(8):bvaa078. doi: 10.1210/jendso/bvaa078. eCollection 2020 Aug 1. J Endocr Soc. 2020. PMID: 32803090 Free PMC article.
-
Nasal delivery of nerve growth factor rescue hypogonadism by up-regulating GnRH and testosterone in aging male mice.EBioMedicine. 2018 Sep;35:295-306. doi: 10.1016/j.ebiom.2018.08.021. Epub 2018 Aug 18. EBioMedicine. 2018. PMID: 30131307 Free PMC article.
-
Apelin Receptor Signaling Protects GT1-7 GnRH Neurons Against Oxidative Stress In Vitro.Cell Mol Neurobiol. 2022 Apr;42(3):753-775. doi: 10.1007/s10571-020-00968-2. Epub 2020 Sep 28. Cell Mol Neurobiol. 2022. PMID: 32989586 Free PMC article.
-
Pooled prevalence of hypothyroidism among Indian females with infertility: A systematic review & meta-analysis.Indian J Med Res. 2024 Jun;159(6):627-636. doi: 10.25259/IJMR_987_23. Indian J Med Res. 2024. PMID: 39382471 Free PMC article. Review.
References
-
- Conn PM, Crowley WF., Jr. Gonadotropin-releasing hormone and its analogs. Annu Rev Med. 1994;45:391–405. - PubMed
-
- Kaiser UB, et al. Studies of gonadotropin-releasing hormone (GnRH) action using GnRH receptor-expressing pituitary cell lines. Endocr Rev. 1997;18:46–70. - PubMed
-
- Smith JT, et al. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–3692. - PubMed
-
- Dierschke DJ, et al. Circhoral oscillations of plasma LH levels in the ovariectomized rhesus monkey. Endocrinology. 1970;87:850–853. - PubMed

