The complement inhibitor FUT-175 suppresses T cell autoreactivity in experimental autoimmune encephalomyelitis
- PMID: 19608865
- PMCID: PMC2715286
- DOI: 10.2353/ajpath.2009.081093
The complement inhibitor FUT-175 suppresses T cell autoreactivity in experimental autoimmune encephalomyelitis
Abstract
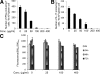
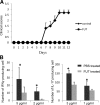
Several recent studies have shown that interacting antigen presenting cells and/or T cells produced complement activation products C5a and C3a, are integrally involved in T-cell activation, and promote the generation of myelin oligodendrocyte glycoprotein (MOG(35-55))-specific interferon-gamma and interleukin-17-producing T cells in experimental autoimmune encephalomyelitis, a rodent model of multiple sclerosis. In this study, we tested whether FUT-175, a clinical pharmaceutical that has been shown to inhibit the formation of C3/C5 convertases, can attenuate myelin-specific T-cell responses, as well as disease severity in experimental autoimmune encephalomyelitis. In vitro, FUT-175 inhibited local C5a/C3a production by antigen presenting cell-T-cell complexes and attenuated MOG(35-55)-specific Th1 and Th17 responses with little nonspecific cytotoxicity. In vivo administration of FUT-175 delayed experimental autoimmune encephalomyelitis disease onset, lowered clinical scores, decreased central nervous system inflammation, and reduced demyelination. The FUT-175-treated mice exhibited decreased numbers of MOG(35-55)-specific interferon-gamma- and interleukin-17-producing T cells. In addition, results from the FUT-175 treatment of naive recipients of adoptively transferred splenocytes from MOG(35-55)-immunized mice suggested that the effect of FUT-175 was on MOG-specific cellular responses and not on anti-MOG antibodies. These results argue that complement regulators, which inhibit C5a/C3a production, may have therapeutic efficacy in multiple sclerosis and in other clinical conditions in which T cells drive disease pathogenesis.
Figures


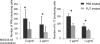

Similar articles
-
IFN-gamma and IL-17 production in experimental autoimmune encephalomyelitis depends on local APC-T cell complement production.J Immunol. 2008 May 1;180(9):5882-9. doi: 10.4049/jimmunol.180.9.5882. J Immunol. 2008. PMID: 18424707 Free PMC article.
-
T cell-depleted splenocytes from mice pre-immunized with neuroantigen in incomplete Freund's adjuvant involved in protection from experimental autoimmune encephalomyelitis.Immunol Lett. 2014 Jan-Feb;157(1-2):38-44. doi: 10.1016/j.imlet.2013.11.001. Epub 2013 Nov 9. Immunol Lett. 2014. PMID: 24220208
-
FUT-175 as a potent inhibitor of C5/C3 convertase activity for production of C5a and C3a.Immunol Lett. 1991 Jan;27(1):49-52. doi: 10.1016/0165-2478(91)90243-4. Immunol Lett. 1991. PMID: 2019419
-
T- and B-cell responses to myelin oligodendrocyte glycoprotein in experimental autoimmune encephalomyelitis and multiple sclerosis.Glia. 2001 Nov;36(2):220-34. doi: 10.1002/glia.1111. Glia. 2001. PMID: 11596130 Review.
-
Myelin oligodendrocyte glycoprotein: a novel candidate autoantigen in multiple sclerosis.J Mol Med (Berl). 1997 Feb;75(2):77-88. doi: 10.1007/s001090050092. J Mol Med (Berl). 1997. PMID: 9083925 Review.
Cited by
-
New insights of an old defense system: structure, function, and clinical relevance of the complement system.Mol Med. 2011 Mar-Apr;17(3-4):317-29. doi: 10.2119/molmed.2010.00149. Epub 2010 Oct 29. Mol Med. 2011. PMID: 21046060 Free PMC article. Review.
-
Nafamostat mesylate, a serine protease inhibitor, demonstrates novel antimicrobial properties and effectiveness in Chlamydia-induced arthritis.Arthritis Res Ther. 2012 Jun 20;14(3):R150. doi: 10.1186/ar3886. Arthritis Res Ther. 2012. PMID: 22716645 Free PMC article.
-
Anti-inflammatory Agents: Present and Future.Cell. 2010 Mar 19;140(6):935-50. doi: 10.1016/j.cell.2010.02.043. Cell. 2010. PMID: 20303881 Free PMC article. Review.
-
Nafamostat mesylate attenuates the pathophysiologic sequelae of neurovascular ischemia.Neural Regen Res. 2020 Dec;15(12):2217-2234. doi: 10.4103/1673-5374.284981. Neural Regen Res. 2020. Retraction in: Neural Regen Res. 2022 May;17(5):958. doi: 10.4103/1673-5374.295353. PMID: 32594033 Free PMC article. Retracted. Review.
-
Complement activation in the injured central nervous system: another dual-edged sword?J Neuroinflammation. 2012 Jun 21;9:137. doi: 10.1186/1742-2094-9-137. J Neuroinflammation. 2012. PMID: 22721265 Free PMC article. Review.
References
-
- Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. - PubMed
-
- Wolinsky JS. The use of glatiramer acetate in the treatment of multiple sclerosis. Adv Neurol. 2006;98:273–292. - PubMed
-
- Morrissey SP, Le Page E, Edan G. Mitoxantrone in the treatment of multiple sclerosis. Int MSJ. 2005;12:74–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

