Removal of maternal retinoic acid by embryonic CYP26 is required for correct Nodal expression during early embryonic patterning
- PMID: 19605690
- PMCID: PMC2714714
- DOI: 10.1101/gad.1776209
Removal of maternal retinoic acid by embryonic CYP26 is required for correct Nodal expression during early embryonic patterning
Abstract
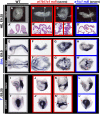
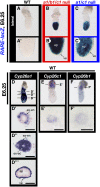
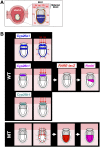
The abundance of retinoic acid (RA) is determined by the balance between its synthesis by retinaldehyde dehydrogenase (RALDH) and its degradation by CYP26. In particular, the dynamic expression of three CYP26 genes controls the regional level of RA within the body. Pregastrulation mouse embryos express CYP26 but not RALDH. We now show that mice lacking all three CYP26 genes manifest duplication of the body axis as a result of expansion of the Nodal expression domain throughout the epiblast. Mouse Nodal was found to contain an RA-responsive element in intron 1 that is highly conserved among mammals. In the absence of CYP26, maternally derived RA activates Nodal expression in the entire epiblast of pregastrulation embryos via this element. These observations suggest that maternal RA must be removed by embryonic CYP26 for correct Nodal expression during embryonic patterning.
Figures





Similar articles
-
The retinoic acid-inactivating enzyme CYP26 is essential for establishing an uneven distribution of retinoic acid along the anterio-posterior axis within the mouse embryo.Genes Dev. 2001 Jan 15;15(2):213-25. doi: 10.1101/gad.851501. Genes Dev. 2001. PMID: 11157777 Free PMC article.
-
Complementary domains of retinoic acid production and degradation in the early chick embryo.Dev Biol. 1999 Dec 1;216(1):282-96. doi: 10.1006/dbio.1999.9487. Dev Biol. 1999. PMID: 10588879
-
Cyp26 enzymes generate the retinoic acid response pattern necessary for hindbrain development.Development. 2007 Jan;134(1):177-87. doi: 10.1242/dev.02706. Development. 2007. PMID: 17164423 Free PMC article.
-
Heads or tails? Retinoic acid will decide.Bioessays. 1999 Oct;21(10):809-12. doi: 10.1002/(SICI)1521-1878(199910)21:10<809::AID-BIES2>3.0.CO;2-0. Bioessays. 1999. PMID: 10497330 Review.
-
The role of CYP26 enzymes in retinoic acid clearance.Expert Opin Drug Metab Toxicol. 2009 Aug;5(8):875-86. doi: 10.1517/17425250903032681. Expert Opin Drug Metab Toxicol. 2009. PMID: 19519282 Free PMC article. Review.
Cited by
-
Mesoderm patterning by a dynamic gradient of retinoic acid signalling.Philos Trans R Soc Lond B Biol Sci. 2020 Oct 12;375(1809):20190556. doi: 10.1098/rstb.2019.0556. Epub 2020 Aug 24. Philos Trans R Soc Lond B Biol Sci. 2020. PMID: 32829679 Free PMC article. Review.
-
Focal facial dermal dysplasia, type IV, is caused by mutations in CYP26C1.Hum Mol Genet. 2013 Feb 15;22(4):696-703. doi: 10.1093/hmg/dds477. Epub 2012 Nov 16. Hum Mol Genet. 2013. PMID: 23161670 Free PMC article.
-
Generating retinoic acid gradients by local degradation during craniofacial development: One cell's cue is another cell's poison.Genesis. 2018 Feb;56(2):10.1002/dvg.23091. doi: 10.1002/dvg.23091. Epub 2018 Jan 25. Genesis. 2018. PMID: 29330906 Free PMC article. Review.
-
Genetic contribution of retinoid-related genes to neural tube defects.Hum Mutat. 2018 Apr;39(4):550-562. doi: 10.1002/humu.23397. Epub 2018 Jan 19. Hum Mutat. 2018. PMID: 29297599 Free PMC article.
-
SHH propagates distal limb bud development by enhancing CYP26B1-mediated retinoic acid clearance via AER-FGF signalling.Development. 2011 May;138(10):1913-23. doi: 10.1242/dev.063966. Epub 2011 Apr 6. Development. 2011. PMID: 21471156 Free PMC article.
References
-
- Abu-Abed S, MacLean G, Fraulob V, Chambon P, Petkovich M, Dolle P. Differential expression of the retinoic acid-metabolizing enzymes CYP26A1 and CYP26B1 during murine organogenesis. Mech Dev. 2002;110:173–177. - PubMed
-
- Brennan J, Lu CC, Norris DP, Rodriguez TA, Beddington RS, Robertson EJ. Nodal signalling in the epiblast patterns the early mouse embryo. Nature. 2001;411:965–969. - PubMed
-
- Collignon J, Varlet I, Robertson EJ. Relationship between asymmetric nodal expression and the direction of embryonic turning. Nature. 1996;381:155–158. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
