FAK phosphorylation by ERK primes ras-induced tyrosine dephosphorylation of FAK mediated by PIN1 and PTP-PEST
- PMID: 19595712
- PMCID: PMC2715139
- DOI: 10.1016/j.molcel.2009.06.013
FAK phosphorylation by ERK primes ras-induced tyrosine dephosphorylation of FAK mediated by PIN1 and PTP-PEST
Abstract
Activated Ras has been found in many types of cancer. However, the mechanism underlying Ras-promoted tumor metastasis remains unclear. We demonstrate here that activated Ras induces tyrosine dephosphorylation and inhibition of FAK mediated by the Ras downstream Fgd1-Cdc42-PAK1-MEK-ERK signaling cascade. ERK phosphorylates FAK S910 and recruits PIN1 and PTP-PEST, which colocalize with FAK at the lamellipodia of migrating cells. PIN1 binding and prolyl isomerization of FAK cause PTP-PEST to interact with and dephosphorylate FAK Y397. Inhibition of FAK mediated by this signal relay promotes Ras-induced cell migration, invasion, and metastasis. These findings uncover the importance of sequential modification of FAK-by serine phosphorylation, isomerization, and tyrosine dephosphorylation--in the regulation of FAK activity and, thereby, in Ras-related tumor metastasis.
Figures
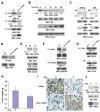


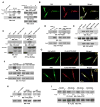

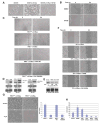

Comment in
-
Ras and the FAK paradox.Mol Cell. 2009 Jul 31;35(2):141-2. doi: 10.1016/j.molcel.2009.07.005. Mol Cell. 2009. PMID: 19647511 Free PMC article.
Similar articles
-
Ras-induced and extracellular signal-regulated kinase 1 and 2 phosphorylation-dependent isomerization of protein tyrosine phosphatase (PTP)-PEST by PIN1 promotes FAK dephosphorylation by PTP-PEST.Mol Cell Biol. 2011 Nov;31(21):4258-69. doi: 10.1128/MCB.05547-11. Epub 2011 Aug 29. Mol Cell Biol. 2011. PMID: 21876001 Free PMC article.
-
Prolactin-induced PAK1 tyrosyl phosphorylation promotes FAK dephosphorylation, breast cancer cell motility, invasion and metastasis.BMC Cell Biol. 2016 Aug 20;17(1):31. doi: 10.1186/s12860-016-0109-5. BMC Cell Biol. 2016. PMID: 27542844 Free PMC article.
-
Activation of focal adhesion kinase enhances the adhesion and invasion of pancreatic cancer cells via extracellular signal-regulated kinase-1/2 signaling pathway activation.Mol Cancer. 2005 Oct 6;4:37. doi: 10.1186/1476-4598-4-37. Mol Cancer. 2005. PMID: 16209712 Free PMC article.
-
Phosphorylation of focal adhesion kinase at tyrosine 861 is crucial for Ras transformation of fibroblasts.J Biol Chem. 2004 Jul 9;279(28):29060-5. doi: 10.1074/jbc.M401183200. Epub 2004 May 3. J Biol Chem. 2004. PMID: 15123632
-
Regulation of tumor cell migration by protein tyrosine phosphatase (PTP)-proline-, glutamate-, serine-,and threonine-rich sequence (PEST).Chin J Cancer. 2013 Feb;32(2):75-83. doi: 10.5732/cjc.012.10084. Epub 2012 Dec 7. Chin J Cancer. 2013. PMID: 23237212 Free PMC article. Review.
Cited by
-
Targeting Pin1 for Modulation of Cell Motility and Cancer Therapy.Biomedicines. 2021 Mar 31;9(4):359. doi: 10.3390/biomedicines9040359. Biomedicines. 2021. PMID: 33807199 Free PMC article. Review.
-
Mutations and deregulation of Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR cascades which alter therapy response.Oncotarget. 2012 Sep;3(9):954-87. doi: 10.18632/oncotarget.652. Oncotarget. 2012. PMID: 23006971 Free PMC article. Review.
-
Targeting Focal Adhesion Kinase Using Inhibitors of Protein-Protein Interactions.Cancers (Basel). 2018 Aug 21;10(9):278. doi: 10.3390/cancers10090278. Cancers (Basel). 2018. PMID: 30134553 Free PMC article. Review.
-
IAP regulation of metastasis.Cancer Cell. 2010 Jan 19;17(1):53-64. doi: 10.1016/j.ccr.2009.11.021. Cancer Cell. 2010. PMID: 20129247 Free PMC article.
-
Characterization of the Src-regulated kinome identifies SGK1 as a key mediator of Src-induced transformation.Nat Commun. 2019 Jan 17;10(1):296. doi: 10.1038/s41467-018-08154-1. Nat Commun. 2019. PMID: 30655532 Free PMC article.
References
-
- Ayaki M, Komatsu K, Mukai M, Murata K, Kameyama M, Ishiguro S, Miyoshi J, Tatsuta M, Nakamura H. Reduced expression of focal adhesion kinase in liver metastases compared with matched primary human colorectal adenocarcinomas. Clin Cancer Res. 2001;7:3106–3112. - PubMed
-
- Ayala I, Giacchetti G, Caldieri G, Attanasio F, Mariggio S, Tete S, Polishchuk R, Castronovo V, Buccione R. Faciogenital dysplasia protein Fgd1 regulates invadopodia biogenesis and extracellular matrix degradation and is up-regulated in prostate and breast cancer. Cancer research. 2009;69:747–752. - PubMed
-
- Basson MD, Sanders MA, Gomez R, Hatfield J, Vanderheide R, Thamilselvan V, Zhang J, Walsh MF. Focal adhesion kinase protein levels in gut epithelial motility. American journal of physiology. 2006;291:G491–499. - PubMed
-
- Beeser A, Jaffer ZM, Hofmann C, Chernoff J. Role of group A p21-activated kinases in activation of extracellular-regulated kinase by growth factors. The Journal of biological chemistry. 2005;280:36609–36615. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

