Self-assembled hydrogels from poly[N-(2-hydroxypropyl)methacrylamide] grafted with beta-sheet peptides
- PMID: 19591463
- PMCID: PMC2737061
- DOI: 10.1021/bm9005084
Self-assembled hydrogels from poly[N-(2-hydroxypropyl)methacrylamide] grafted with beta-sheet peptides
Abstract
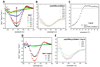
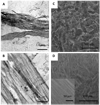
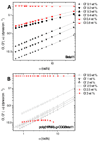
A new hybrid hydrogel based on poly[N-(2-hydroxypropyl)methacrylamide] grafted with a beta-sheet peptide, Beta11, was designed. Circular dichroism spectroscopy indicated that the folding ability of beta-sheet peptide was retained in the hybrid system, whereas the sensitivity of the peptide toward temperature and pH variations was hindered. The polymer backbone also prevented the twisting of the fibrils that resulted from the antiparallel arrangement of the beta-strands, as proved by Fourier transform infrared spectroscopy. Thioflavin T binding experiments and transmission electron microscopy showed fibril formation with minimal lateral aggregation. As a consequence, the graft copolymer self-assembled into a hydrogel in aqueous environment. This process was mediated by association of beta-sheet domains. Scanning electron microscopy revealed a particular morphology of the network characterized by long-range order and uniformly aligned lamellae. Microrheology results confirmed that concentration-dependent gelation occurred.
Figures




Similar articles
-
Hybrid hydrogels self-assembled from graft copolymers containing complementary β-sheets as hydroxyapatite nucleation scaffolds.Biomaterials. 2011 Aug;32(23):5341-53. doi: 10.1016/j.biomaterials.2011.04.014. Epub 2011 May 5. Biomaterials. 2011. PMID: 21549421 Free PMC article.
-
Self-Healing, Self-Assembled β-Sheet Peptide-Poly(γ-glutamic acid) Hybrid Hydrogels.J Am Chem Soc. 2017 May 31;139(21):7250-7255. doi: 10.1021/jacs.7b00528. Epub 2017 May 19. J Am Chem Soc. 2017. PMID: 28525280 Free PMC article.
-
Refolding hydrogels self-assembled from N-(2-hydroxypropyl)methacrylamide graft copolymers by antiparallel coiled-coil formation.Biomacromolecules. 2006 Apr;7(4):1187-95. doi: 10.1021/bm051002k. Biomacromolecules. 2006. PMID: 16602737 Free PMC article.
-
Existence of different structural intermediates on the fibrillation pathway of human serum albumin.Biophys J. 2009 Mar 18;96(6):2353-70. doi: 10.1016/j.bpj.2008.12.3901. Biophys J. 2009. PMID: 19289061 Free PMC article.
-
Identification of a novel human islet amyloid polypeptide beta-sheet domain and factors influencing fibrillogenesis.J Mol Biol. 2001 May 4;308(3):515-25. doi: 10.1006/jmbi.2001.4593. J Mol Biol. 2001. PMID: 11327784
Cited by
-
Hybrid hydrogels self-assembled from graft copolymers containing complementary β-sheets as hydroxyapatite nucleation scaffolds.Biomaterials. 2011 Aug;32(23):5341-53. doi: 10.1016/j.biomaterials.2011.04.014. Epub 2011 May 5. Biomaterials. 2011. PMID: 21549421 Free PMC article.
-
Self-Healing, Self-Assembled β-Sheet Peptide-Poly(γ-glutamic acid) Hybrid Hydrogels.J Am Chem Soc. 2017 May 31;139(21):7250-7255. doi: 10.1021/jacs.7b00528. Epub 2017 May 19. J Am Chem Soc. 2017. PMID: 28525280 Free PMC article.
-
Synthesis and characterization of enzymatically degradable PEG-based peptide-containing hydrogels.Macromol Biosci. 2010 Apr 8;10(4):445-54. doi: 10.1002/mabi.200900295. Macromol Biosci. 2010. PMID: 20146210 Free PMC article.
-
POLYMERIC BIOMATERIALS AND NANOMEDICINES.J Drug Deliv Sci Technol. 2015 Dec 1;30(Pt B):318-330. doi: 10.1016/j.jddst.2015.05.012. J Drug Deliv Sci Technol. 2015. PMID: 26688694 Free PMC article.
-
Hybrid polymeric hydrogels via peptide nucleic acid (PNA)/DNA complexation.J Control Release. 2015 Dec 28;220(Pt B):608-16. doi: 10.1016/j.jconrel.2015.09.035. Epub 2015 Sep 21. J Control Release. 2015. PMID: 26394062 Free PMC article.
References
-
- Hentschel J, Krause E, Börner HG. J. Am. Chem. Soc. 2006;128:7722–7723. - PubMed
-
- Börner HG, Smarsly BM, Hentschel J, Rank A, Schubert R, Geng Y, Discher DE, Hellweg T, Brandt A. Macromolecules. 2008;41:1430–1437.
-
- Eckhardt D, Groenewolt M, Krause E, Börner HG. Chem. Commun. 2005:2814–2816. - PubMed
-
- Rathore O, Sogah DY. J. Am. Chem. Soc. 2001;123:5231–5239. - PubMed
-
- Rösler A, Klok H-A, Hamley IW, Castelletto V, Mykhaylyk OO. Biomacromolecules. 2003;4:859–863. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

