Mechanisms promoting translocations in editing and switching peripheral B cells
- PMID: 19587764
- PMCID: PMC2907259
- DOI: 10.1038/nature08159
Mechanisms promoting translocations in editing and switching peripheral B cells
Abstract
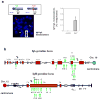
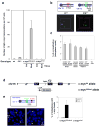
Variable, diversity and joining gene segment (V(D)J) recombination assembles immunoglobulin heavy or light chain (IgH or IgL) variable region exons in developing bone marrow B cells, whereas class switch recombination (CSR) exchanges IgH constant region exons in peripheral B cells. Both processes use directed DNA double-strand breaks (DSBs) repaired by non-homologous end-joining (NHEJ). Errors in either V(D)J recombination or CSR can initiate chromosomal translocations, including oncogenic IgH locus (Igh) to c-myc (also known as Myc) translocations of peripheral B cell lymphomas. Collaboration between these processes has also been proposed to initiate translocations. However, the occurrence of V(D)J recombination in peripheral B cells is controversial. Here we show that activated NHEJ-deficient splenic B cells accumulate V(D)J-recombination-associated breaks at the lambda IgL locus (Igl), as well as CSR-associated Igh breaks, often in the same cell. Moreover, Igl and Igh breaks are frequently joined to form translocations, a phenomenon associated with specific Igh-Igl co-localization. Igh and c-myc also co-localize in these cells; correspondingly, the introduction of frequent c-myc DSBs robustly promotes Igh-c-myc translocations. Our studies show peripheral B cells that attempt secondary V(D)J recombination, and determine a role for mechanistic factors in promoting recurrent translocations in tumours.
Figures


Comment in
-
Immunology: B cells break the rules.Nature. 2009 Jul 9;460(7252):184-6. doi: 10.1038/460184a. Nature. 2009. PMID: 19587755 No abstract available.
Similar articles
-
Long-range oncogenic activation of Igh-c-myc translocations by the Igh 3' regulatory region.Nature. 2009 Dec 10;462(7274):803-7. doi: 10.1038/nature08633. Nature. 2009. PMID: 20010689 Free PMC article.
-
IgH class switching and translocations use a robust non-classical end-joining pathway.Nature. 2007 Sep 27;449(7161):478-82. doi: 10.1038/nature06020. Epub 2007 Aug 22. Nature. 2007. PMID: 17713479
-
Immunology: B cells break the rules.Nature. 2009 Jul 9;460(7252):184-6. doi: 10.1038/460184a. Nature. 2009. PMID: 19587755 No abstract available.
-
Mechanism and control of V(D)J recombination versus class switch recombination: similarities and differences.Adv Immunol. 2005;86:43-112. doi: 10.1016/S0065-2776(04)86002-4. Adv Immunol. 2005. PMID: 15705419 Review.
-
Regulation of immunoglobulin class-switch recombination: choreography of noncoding transcription, targeted DNA deamination, and long-range DNA repair.Adv Immunol. 2014;122:1-57. doi: 10.1016/B978-0-12-800267-4.00001-8. Adv Immunol. 2014. PMID: 24507154 Free PMC article. Review.
Cited by
-
Robust chromosomal DNA repair via alternative end-joining in the absence of X-ray repair cross-complementing protein 1 (XRCC1).Proc Natl Acad Sci U S A. 2012 Feb 14;109(7):2473-8. doi: 10.1073/pnas.1121470109. Epub 2012 Jan 30. Proc Natl Acad Sci U S A. 2012. PMID: 22308491 Free PMC article.
-
Delineating Rearrangements in Single Yeast Artificial Chromosomes by Quantitative DNA Fiber Mapping.Open Genomics J. 2009 Oct 9;2:15-23. doi: 10.2174/1875693X00902010015. Open Genomics J. 2009. PMID: 20502619 Free PMC article.
-
Alternative end-joining in BCR gene rearrangements and translocations.Acta Biochim Biophys Sin (Shanghai). 2022 May 25;54(6):782-795. doi: 10.3724/abbs.2022051. Acta Biochim Biophys Sin (Shanghai). 2022. PMID: 35593472 Free PMC article. Review.
-
Mitotic homologous recombination maintains genomic stability and suppresses tumorigenesis.Nat Rev Mol Cell Biol. 2010 Mar;11(3):196-207. doi: 10.1038/nrm2851. Nat Rev Mol Cell Biol. 2010. PMID: 20177395 Free PMC article. Review.
-
KSHV induces immunoglobulin rearrangements in mature B lymphocytes.PLoS Pathog. 2018 Apr 16;14(4):e1006967. doi: 10.1371/journal.ppat.1006967. eCollection 2018 Apr. PLoS Pathog. 2018. PMID: 29659614 Free PMC article.
References
-
- Jung D, Alt FW. Unraveling V(D)J recombination; insights into gene regulation. Cell. 2004;116:299–311. - PubMed
-
- Rooney S, Chaudhuri J, Alt FW. The role of the non-homologous end-joining pathway in lymphocyte development. Immunol Rev. 2004;200:115–31. - PubMed
-
- Bassing CH, Swat W, Alt FW. The mechanism and regulation of chromosomal V(D)J recombination. Cell. 2002;109(Suppl):S45–55. - PubMed
-
- Gorman JR, Alt FW. Regulation of immunoglobulin light chain isotype expression. Adv Immunol. 1998;69:113–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 CA092625-010001/CA/NCI NIH HHS/United States
- 5P01CA92625/CA/NCI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- T32 CA009382/CA/NCI NIH HHS/United States
- P01 CA092625-080006/CA/NCI NIH HHS/United States
- T32 CA009382-27/CA/NCI NIH HHS/United States
- P01 CA092625-060006/CA/NCI NIH HHS/United States
- P01 CA092625-070006/CA/NCI NIH HHS/United States
- P01 CA092625/CA/NCI NIH HHS/United States
- P01 CA092625-020001/CA/NCI NIH HHS/United States
- T32 CA009382-28/CA/NCI NIH HHS/United States
- P01 CA092625-090006/CA/NCI NIH HHS/United States
- R01 AI077595/AI/NIAID NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
- R37 AI077595/AI/NIAID NIH HHS/United States
- R01 AI077595-02/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

