Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells
- PMID: 19587053
- PMCID: PMC2738247
- DOI: 10.1128/JVI.00639-09
Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells
Abstract
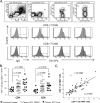
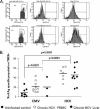
A number of emerging molecules and pathways have been implicated in mediating the T-cell exhaustion characteristic of chronic viral infection. Not all dysfunctional T cells express PD-1, nor are they all rescued by blockade of the PD-1/PD-1 ligand pathway. In this study, we characterize the expression of T-cell immunoglobulin and mucin domain-containing protein 3 (Tim-3) in chronic hepatitis C infection. For the first time, we found that Tim-3 expression is increased on CD4(+) and CD8(+) T cells in chronic hepatitis C virus (HCV) infection. The proportion of dually PD-1/Tim-3-expressing cells is greatest in liver-resident T cells, significantly more so in HCV-specific than in cytomegalovirus-specific cytotoxic T lymphocytes. Tim-3 expression correlates with a dysfunctional and senescent phenotype (CD127(low) CD57(high)), a central rather than effector memory profile (CD45RA(negative) CCR7(high)), and reduced Th1/Tc1 cytokine production. We also demonstrate the ability to enhance T-cell proliferation and gamma interferon production in response to HCV-specific antigens by blocking the Tim-3-Tim-3 ligand interaction. These findings have implications for the development of novel immunotherapeutic approaches to this common viral infection.
Figures
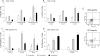




Similar articles
-
HCV-specific T cells in HCV/HIV co-infection show elevated frequencies of dual Tim-3/PD-1 expression that correlate with liver disease progression.Eur J Immunol. 2010 Sep;40(9):2493-505. doi: 10.1002/eji.201040340. Eur J Immunol. 2010. PMID: 20623550
-
Splenic CD4+ and CD8+ T-cells highly expressed PD-1 and Tim-3 in cirrhotic patients with HCV infection and portal hypertension.Int J Immunopathol Pharmacol. 2021 Jan-Dec;35:20587384211061051. doi: 10.1177/20587384211061051. Int J Immunopathol Pharmacol. 2021. PMID: 34930041 Free PMC article.
-
Upregulation of the Tim-3/galectin-9 pathway of T cell exhaustion in chronic hepatitis B virus infection.PLoS One. 2012;7(10):e47648. doi: 10.1371/journal.pone.0047648. Epub 2012 Oct 24. PLoS One. 2012. PMID: 23112829 Free PMC article.
-
Costimulatory molecule programmed death-1 in the cytotoxic response during chronic hepatitis C.World J Gastroenterol. 2009 Nov 7;15(41):5129-40. doi: 10.3748/wjg.15.5129. World J Gastroenterol. 2009. PMID: 19891011 Free PMC article. Review.
-
Too much of a good thing? Tim-3 and TCR signaling in T cell exhaustion.J Immunol. 2014 Aug 15;193(4):1525-30. doi: 10.4049/jimmunol.1400557. J Immunol. 2014. PMID: 25086175 Free PMC article. Review.
Cited by
-
Tim-3/galectin-9 regulate the homeostasis of hepatic NKT cells in a murine model of nonalcoholic fatty liver disease.J Immunol. 2013 Feb 15;190(4):1788-96. doi: 10.4049/jimmunol.1202814. Epub 2013 Jan 7. J Immunol. 2013. PMID: 23296703 Free PMC article.
-
Negative CD4 + TIM-3 signaling confers resistance against cold preservation damage in mouse liver transplantation.Am J Transplant. 2015 Apr;15(4):954-964. doi: 10.1111/ajt.13067. Epub 2015 Feb 12. Am J Transplant. 2015. PMID: 25676534 Free PMC article.
-
Exploring the Mechanisms Underlying the Cardiotoxic Effects of Immune Checkpoint Inhibitor Therapies.Vaccines (Basel). 2022 Mar 31;10(4):540. doi: 10.3390/vaccines10040540. Vaccines (Basel). 2022. PMID: 35455289 Free PMC article. Review.
-
Tim3 binding to galectin-9 stimulates antimicrobial immunity.J Exp Med. 2010 Oct 25;207(11):2343-54. doi: 10.1084/jem.20100687. Epub 2010 Oct 11. J Exp Med. 2010. PMID: 20937702 Free PMC article.
-
Combined Use of Immune Checkpoint Inhibitors and Phytochemicals as a Novel Therapeutic Strategy against Cancer.J Cancer. 2023 Jul 24;14(12):2315-2328. doi: 10.7150/jca.85966. eCollection 2023. J Cancer. 2023. PMID: 37576404 Free PMC article. Review.
References
-
- Appay, V., P. R. Dunbar, M. Callan, P. Klenerman, G. M. A. Gillespie, L. Papagno, G. S. Ogg, A. King, F. Lechner, C. A. Spina, S. Little, D. V. Havlir, D. D. Richman, N. Gruener, G. Pape, A. Waters, P. Easterbrook, M. Salio, V. Cerundolo, A. J., McMichael, and S. L. Rowland-Jones. 2002. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 8:379-385. - PubMed
-
- Baran, J., D. Kowalczyk, M. Ozóg, and M. Zembala. 2001. Three-color flow cytometry detection of intracellular cytokines in peripheral blood mononuclear cells: comparative analysis of phorbol myristate acetate-ionomycin and phytohemagglutinin stimulation. Clin. Diagn. Lab. Immunol. 8:303-313. - PMC - PubMed
-
- Burton, J. R., Jr., J. Klarquist, K. Im, S. Smyk-Pearson, L. Golden-Mason, N. Castelblanco, N. Terrault, H. R. Rosen, and the Virahep-C Study Group. 2008. Prospective analysis of effector and regulatory CD4+ T cells in chronic HCV patients undergoing combination antiviral therapy. J. Hepatol. 49:329-338. - PubMed
-
- D'Souza, M., A. P. Fontenot, D. G. Mack, C. Lozupone, S. Dillon, A. Meditz, C. C. Wilson, E. Connick, and B. E. Palmer. 2007. Programmed death 1 expression on HIV-specific CD4+ T cells is driven by viral replication and associated with T cell dysfunction. J. Immunol. 179:1979-1987. - PubMed
-
- Golden-Mason, L., J. R. Burton, Jr., N. Castelblanco, J. Klarquist, S. Benlloch, C. Wang, and H. R. Rosen. 2006. Loss of IL-7 receptor alpha-chain (CD127) expression in acute HCV infection associated with viral persistence. Hepatology 44:1098-1109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

