Physical interaction between replication protein A (RPA) and MRN: involvement of RPA2 phosphorylation and the N-terminus of RPA1
- PMID: 19586055
- PMCID: PMC2737085
- DOI: 10.1021/bi900694p
Physical interaction between replication protein A (RPA) and MRN: involvement of RPA2 phosphorylation and the N-terminus of RPA1
Abstract
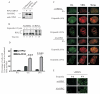
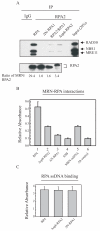
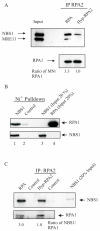
Replication protein A (RPA) is a heterotrimeric protein consisting of RPA1, RPA2, and RPA3 subunits that binds to single-stranded DNA (ssDNA) with high affinity. The response to replication stress requires the recruitment of RPA and the MRE11-RAD50-NBS1 (MRN) complex. RPA bound to ssDNA stabilizes stalled replication forks by recruiting checkpoint proteins involved in fork stabilization. MRN can bind DNA structures encountered at stalled or collapsed replication forks, such as ssDNA-double-stranded DNA (dsDNA) junctions or breaks, and promote the restart of DNA replication. Here, we demonstrate that RPA2 phosphorylation regulates the assembly of DNA damage-induced RPA and MRN foci. Using purified proteins, we observe a direct interaction between RPA with both NBS1 and MRE11. By utilizing RPA bound to ssDNA, we demonstrate that substituting RPA with phosphorylated RPA or a phosphomimetic weakens the interaction with the MRN complex. Also, the N-terminus of RPA1 is a critical component of the RPA-MRN protein-protein interaction. Deletion of the N-terminal oligonucleotide-oligosaccharide binding fold (OB-fold) of RPA1 abrogates interactions of RPA with MRN and individual proteins of the MRN complex. Further identification of residues critical for MRN binding in the N-terminus of RPA1 shows that substitution of Arg31 and Arg41 with alanines disrupts the RPA-MRN interaction and alters cell cycle progression in response to DNA damage. Thus, the N-terminus of RPA1 and phosphorylation of RPA2 regulate RPA-MRN interactions and are important in the response to DNA damage.
Figures



Similar articles
-
Replication protein A and the Mre11.Rad50.Nbs1 complex co-localize and interact at sites of stalled replication forks.J Biol Chem. 2004 Aug 13;279(33):34802-10. doi: 10.1074/jbc.M404750200. Epub 2004 Jun 4. J Biol Chem. 2004. PMID: 15180989
-
DNA lesion-specific co-localization of the Mre11/Rad50/Nbs1 (MRN) complex and replication protein A (RPA) to repair foci.J Biol Chem. 2005 Apr 1;280(13):12927-34. doi: 10.1074/jbc.M414391200. Epub 2005 Jan 14. J Biol Chem. 2005. PMID: 15653682
-
Replication protein A is required for etoposide-induced assembly of MRE11/RAD50/NBS1 complex repair foci.Cell Cycle. 2007 Oct 1;6(19):2408-16. doi: 10.4161/cc.6.19.4773. Epub 2007 Jul 20. Cell Cycle. 2007. PMID: 17700070
-
RPA-coated single-stranded DNA as a platform for post-translational modifications in the DNA damage response.Cell Res. 2015 Jan;25(1):9-23. doi: 10.1038/cr.2014.147. Epub 2014 Nov 18. Cell Res. 2015. PMID: 25403473 Free PMC article. Review.
-
Replication protein A, the laxative that keeps DNA regular: The importance of RPA phosphorylation in maintaining genome stability.Semin Cell Dev Biol. 2019 Feb;86:112-120. doi: 10.1016/j.semcdb.2018.04.005. Epub 2018 Nov 13. Semin Cell Dev Biol. 2019. PMID: 29665433 Review.
Cited by
-
The SNM1B/APOLLO DNA nuclease functions in resolution of replication stress and maintenance of common fragile site stability.Hum Mol Genet. 2013 Dec 15;22(24):4901-13. doi: 10.1093/hmg/ddt340. Epub 2013 Jul 17. Hum Mol Genet. 2013. PMID: 23863462 Free PMC article.
-
Two distinct modes of ATR activation orchestrated by Rad17 and Nbs1.Cell Rep. 2013 May 30;3(5):1651-62. doi: 10.1016/j.celrep.2013.04.018. Epub 2013 May 16. Cell Rep. 2013. PMID: 23684611 Free PMC article.
-
Ubiquitylation at the Fork: Making and Breaking Chains to Complete DNA Replication.Int J Mol Sci. 2018 Sep 25;19(10):2909. doi: 10.3390/ijms19102909. Int J Mol Sci. 2018. PMID: 30257459 Free PMC article. Review.
-
The Multiple Faces of the MRN Complex: Roles in Medulloblastoma and Beyond.Cancers (Basel). 2023 Jul 13;15(14):3599. doi: 10.3390/cancers15143599. Cancers (Basel). 2023. PMID: 37509263 Free PMC article. Review.
-
More forks on the road to replication stress recovery.J Mol Cell Biol. 2011 Feb;3(1):4-12. doi: 10.1093/jmcb/mjq049. J Mol Cell Biol. 2011. PMID: 21278446 Free PMC article. Review.
References
-
- Park MS, Ludwig DL, Stigger E, Lee SH. Physical interaction between human RAD52 and RPA is required for homologous recombination in mammalian cells. J.Biol Chem. 1996;271:18996–19000. - PubMed
-
- Perrault R, Cheong N, Wang H, Wang H, Iliakis G. RPA facilitates rejoining of DNA double-strand breaks in an in vitro assay utilizing genomic DNA as substrate. Int J Radiat Biol. 2001;77:593–607. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

