DNA promoter hypermethylation of p16 and APC predicts neoplastic progression in Barrett's esophagus
- PMID: 19584833
- PMCID: PMC3090447
- DOI: 10.1038/ajg.2009.300
DNA promoter hypermethylation of p16 and APC predicts neoplastic progression in Barrett's esophagus
Abstract
Objectives: Prediction of progression to cancer in patients with Barrett's esophagus (BE) is difficult using current techniques. We determined whether DNA promoter hypermethylation of genes frequently methylated in esophageal adenocarcinoma (p16 and APC) could be used as predictors of progression in BE.
Methods: We first performed a cross-sectional study to evaluate the prevalence of gene hypermethylation in biopsies from patients with normal esophagus (n=17), BE (n=102), and adenocarcinoma (n=42). We then performed a nested case-control study comparing gene hypermethylation in BE patients who progressed from baseline pathology to high-grade dysplasia or cancer (n=7) vs. patients who did not progress (n=50).
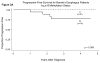
Results: None of the patients with normal esophagus had p16 or APC hypermethylation. Hypermethylation was prevalent in BE without dysplasia or low-grade dysplasia (p16=31% and APC=50%; P<0.01) and high-grade dysplasia or adenocarcinoma (p16=54% and APC=68%; P<0.001) compared with normal esophagus (not detected). Patients who progressed from baseline pathology to high-grade dysplasia or cancer had higher prevalence of hypermethylation in their initial esophagus biopsies compared with those who did not progress for both p16 (100 vs. 33%; P=0.008) and APC (86 vs. 40%; P=0.02). Hypermethylation of both p16 and APC was a strong predictor of subsequent progression to high-grade dysplasia or cancer during a mean follow-up time of 4.1 years (odds ratio (95% confidence interval)=14.97 (1.73,inf), P=0.01). Among patients who were negative for both p16 and APC hypermethylation, none progressed from baseline pathology to high-grade dysplasia or cancer.
Conclusions: Hypermethylation of both p16 and APC strongly predicts progression to high-grade dysplasia or cancer in patients with BE. Absence of p16 and APC hypermethylation is associated with a benign course.
Conflict of interest statement
Figures



Similar articles
-
Hypermethylation of the CDKN2/p16 promoter during neoplastic progression in Barrett's esophagus.Gastroenterology. 1998 Dec;115(6):1381-6. doi: 10.1016/s0016-5085(98)70016-2. Gastroenterology. 1998. PMID: 9834265
-
p16 inactivation by methylation of the CDKN2A promoter occurs early during neoplastic progression in Barrett's esophagus.Gastroenterology. 2002 Apr;122(4):1113-21. doi: 10.1053/gast.2002.32370. Gastroenterology. 2002. PMID: 11910361
-
Inactivation of p16, RUNX3, and HPP1 occurs early in Barrett's-associated neoplastic progression and predicts progression risk.Oncogene. 2005 Jun 9;24(25):4138-48. doi: 10.1038/sj.onc.1208598. Oncogene. 2005. PMID: 15824739
-
The diagnosis and management of Barrett's esophagus.Adv Surg. 1999;33:29-68. Adv Surg. 1999. PMID: 10572561 Review.
-
Predictors of Progression to High-Grade Dysplasia or Adenocarcinoma in Barrett's Esophagus.Gastroenterol Clin North Am. 2015 Jun;44(2):299-315. doi: 10.1016/j.gtc.2015.02.005. Epub 2015 Mar 31. Gastroenterol Clin North Am. 2015. PMID: 26021196 Free PMC article. Review.
Cited by
-
Investigation of early neoplastic transformation and premalignant biology using genetically engineered organoid models.Comput Struct Biotechnol J. 2022 Sep 21;20:5309-5315. doi: 10.1016/j.csbj.2022.09.026. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 36212534 Free PMC article. Review.
-
DNA methylation as an adjunct to histopathology to detect prevalent, inconspicuous dysplasia and early-stage neoplasia in Barrett's esophagus.Clin Cancer Res. 2013 Feb 15;19(4):878-88. doi: 10.1158/1078-0432.CCR-12-2880. Epub 2012 Dec 14. Clin Cancer Res. 2013. PMID: 23243219 Free PMC article.
-
Role of epigenetic alterations in the pathogenesis of Barrett's esophagus and esophageal adenocarcinoma.Int J Clin Exp Pathol. 2012;5(5):382-96. Epub 2012 May 23. Int J Clin Exp Pathol. 2012. PMID: 22808291 Free PMC article. Review.
-
Carcinoma of unknown primary: Molecular tumor board-based therapy.CA Cancer J Clin. 2022 Nov;72(6):510-523. doi: 10.3322/caac.21748. Epub 2022 Aug 25. CA Cancer J Clin. 2022. PMID: 36006378 Free PMC article. No abstract available.
-
Intratumor Epigenetic Heterogeneity-A Panel Gene Methylation Study in Thyroid Cancer.Front Genet. 2021 Sep 3;12:714071. doi: 10.3389/fgene.2021.714071. eCollection 2021. Front Genet. 2021. PMID: 34539742 Free PMC article.
References
-
- Shaheen N, Ransohoff DF. Gastroesophageal reflux, Barrett esophagus, and esophageal cancer: clinical applications. Jama. 2002;287:1982–6. - PubMed
-
- Montgomery E, Goldblum JR, Greenson JK, et al. Dysplasia as a predictive marker for invasive carcinoma in Barrett esophagus: a follow-up study based on 138 cases from a diagnostic variability study. Hum Pathol. 2001;32:379–88. - PubMed
-
- Montgomery E, Bronner MP, Goldblum JR, et al. Reproducibility of the diagnosis of dysplasia in Barrett esophagus: a reaffirmation. Hum Pathol. 2001;32:368–78. - PubMed
-
- Cameron AJ, Ott BJ, Payne WS. The incidence of adenocarcinoma in columnar-lined (Barrett’s) esophagus. N Engl J Med. 1985;313:857–9. - PubMed
-
- Williamson WA, Ellis FH, Jr, Gibb SP, et al. Barrett’s esophagus. Prevalence and incidence of adenocarcinoma. Archives of internal medicine. 1991;151:2212–6. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

