Genetic evidence supporting a critical role of endothelial caveolin-1 during the progression of atherosclerosis
- PMID: 19583953
- PMCID: PMC2735117
- DOI: 10.1016/j.cmet.2009.06.003
Genetic evidence supporting a critical role of endothelial caveolin-1 during the progression of atherosclerosis
Abstract
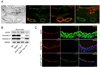
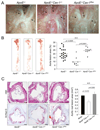
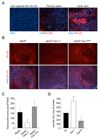
The accumulation of LDL-derived cholesterol in the artery wall is the initiating event that causes atherosclerosis. However, the mechanisms that lead to the initiation of atherosclerosis are still poorly understood. Here, by using endothelial cell-specific transgenesis of the caveolin-1 (Cav-1) gene in mice, we show the critical role of Cav-1 in promoting atherogenesis. Mice were generated lacking Cav-1 and apoE but expressing endothelial-specific Cav-1 in the double knockout background. Genetic ablation of Cav-1 on an apoE knockout background inhibits the progression of atherosclerosis, while re-expression of Cav-1 in the endothelium promotes lesion expansion. Mechanistically, the loss of Cav-1 reduces LDL infiltration into the artery wall, promotes nitric oxide production, and reduces the expression of leukocyte adhesion molecules, effects completely reversed in transgenic mice. In summary, this unique model provides physiological evidence supporting the important role of endothelial Cav-1 expression in regulating the entry of LDL into the vessel wall and the initiation of atherosclerosis.
Figures

Similar articles
-
Endothelial-specific overexpression of caveolin-1 accelerates atherosclerosis in apolipoprotein E-deficient mice.Am J Pathol. 2010 Aug;177(2):998-1003. doi: 10.2353/ajpath.2010.091287. Epub 2010 Jun 25. Am J Pathol. 2010. PMID: 20581061 Free PMC article.
-
PPARgamma1-induced caveolin-1 enhances cholesterol efflux and attenuates atherosclerosis in apolipoprotein E-deficient mice.J Vasc Res. 2010;47(1):69-79. doi: 10.1159/000235927. Epub 2009 Sep 3. J Vasc Res. 2010. PMID: 19729954
-
Role of endothelial cell-derived angptl2 in vascular inflammation leading to endothelial dysfunction and atherosclerosis progression.Arterioscler Thromb Vasc Biol. 2014 Apr;34(4):790-800. doi: 10.1161/ATVBAHA.113.303116. Epub 2014 Feb 13. Arterioscler Thromb Vasc Biol. 2014. PMID: 24526691
-
Leptin promotes neointima formation and smooth muscle cell proliferation via NADPH oxidase activation and signalling in caveolin-rich microdomains.Cardiovasc Res. 2013 Aug 1;99(3):555-65. doi: 10.1093/cvr/cvt126. Epub 2013 May 30. Cardiovasc Res. 2013. PMID: 23723060
-
Cav-1 (Caveolin-1) Deficiency Increases Autophagy in the Endothelium and Attenuates Vascular Inflammation and Atherosclerosis.Arterioscler Thromb Vasc Biol. 2020 Jun;40(6):1510-1522. doi: 10.1161/ATVBAHA.120.314291. Epub 2020 Apr 30. Arterioscler Thromb Vasc Biol. 2020. PMID: 32349535 Free PMC article.
Cited by
-
Polychlorinated biphenyl-induced VCAM-1 expression is attenuated in aortic endothelial cells isolated from caveolin-1 deficient mice.Toxicol Appl Pharmacol. 2010 Jul;246(1-2):74-82. doi: 10.1016/j.taap.2010.04.009. Epub 2010 Apr 18. Toxicol Appl Pharmacol. 2010. PMID: 20406653 Free PMC article.
-
Genome-wide RNAi screen reveals ALK1 mediates LDL uptake and transcytosis in endothelial cells.Nat Commun. 2016 Nov 21;7:13516. doi: 10.1038/ncomms13516. Nat Commun. 2016. PMID: 27869117 Free PMC article.
-
Entry and exit of extracellular vesicles to and from the blood circulation.Nat Nanotechnol. 2024 Jan;19(1):13-20. doi: 10.1038/s41565-023-01522-z. Epub 2023 Dec 18. Nat Nanotechnol. 2024. PMID: 38110531 Free PMC article. Review.
-
Endothelial caveolae and caveolin-1 as key regulators of atherosclerosis.Am J Pathol. 2010 Aug;177(2):544-6. doi: 10.2353/ajpath.2010.100247. Epub 2010 Jun 25. Am J Pathol. 2010. PMID: 20581059 Free PMC article.
-
Diversity of Lipid Function in Atherogenesis: A Focus on Endothelial Mechanobiology.Int J Mol Sci. 2021 Oct 26;22(21):11545. doi: 10.3390/ijms222111545. Int J Mol Sci. 2021. PMID: 34768974 Free PMC article. Review.
References
-
- Busse R, Fleming I. Endothelial dysfunction in atherosclerosis. J Vasc Res. 1996;33:181–194. - PubMed
-
- Cohen AW, Razani B, Wang XB, Combs TP, Williams TM, Scherer PE, Lisanti MP. Caveolin-1-deficient mice show insulin resistance and defective insulin receptor protein expression in adipose tissue. Am J Physiol Cell Physiol. 2003;285:C222–C235. - PubMed
-
- DG LEC, Cogger VC, McCuskey RS, R DEC, Smedsrod B, Sorensen KK, Warren A, Fraser R. Age-related changes in the liver sinusoidal endothelium: a mechanism for dyslipidemia. Ann N Y Acad Sci. 2007;1114:79–87. - PubMed
-
- Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL070295/HL/NHLBI NIH HHS/United States
- R01 HL61371/HL/NHLBI NIH HHS/United States
- R01 HL64793/HL/NHLBI NIH HHS/United States
- R01 HL064793/HL/NHLBI NIH HHS/United States
- N01-HV-28186/HV/NHLBI NIH HHS/United States
- R37 HL061371-11/HL/NHLBI NIH HHS/United States
- R01 HL081190-04/HL/NHLBI NIH HHS/United States
- R01 HL061371/HL/NHLBI NIH HHS/United States
- P01 HL070295-089002/HL/NHLBI NIH HHS/United States
- R37 HL061371/HL/NHLBI NIH HHS/United States
- R01 HL057665/HL/NHLBI NIH HHS/United States
- R01 HL096670/HL/NHLBI NIH HHS/United States
- R01 HL064793-10/HL/NHLBI NIH HHS/United States
- P01 HL70295/HL/NHLBI NIH HHS/United States
- R01 HL57665/HL/NHLBI NIH HHS/United States
- N01HV28186/HV/NHLBI NIH HHS/United States
- R01 HL081190/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

