Nuclear import of the glucocorticoid receptor-hsp90 complex through the nuclear pore complex is mediated by its interaction with Nup62 and importin beta
- PMID: 19581287
- PMCID: PMC2725705
- DOI: 10.1128/MCB.00649-09
Nuclear import of the glucocorticoid receptor-hsp90 complex through the nuclear pore complex is mediated by its interaction with Nup62 and importin beta
Abstract
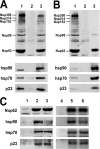
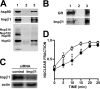
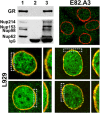
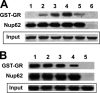
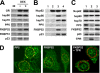
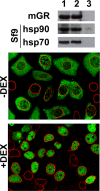
Glucocorticoid receptor (GR) is cytoplasmic in the absence of ligand and localizes to the nucleus after steroid binding. Previous evidence demonstrated that the hsp90-based heterocomplex bound to GR is required for the efficient retrotransport of the receptor to the nuclear compartment. We examined the putative association of GR and its associated chaperone heterocomplex with structures of the nuclear pore. We found that importin beta and the integral nuclear pore glycoprotein Nup62 interact with hsp90, hsp70, p23, and the TPR domain proteins FKBP52 and PP5. Nup62 and GR were able to interact in a more efficient manner when chaperoned by the hsp90-based heterocomplex. Interestingly, the binding of hsp70 and p23 to Nup62 does not require the presence of hsp90, whereas the association of FKBP52 and PP5 is hsp90 dependent, as indicated by the results of experiments where the hsp90 function was disrupted with radicicol. The ability of both FKBP52 and PP5 to interact with Nup62 was abrogated in cells overexpressing the TPR peptide. Importantly, GR cross-linked to the hsp90 heterocomplex was able to translocate to the nucleus in digitonin-permeabilized cells treated with steroid, suggesting that GR could pass through the pore in its untransformed state.
Figures
Similar articles
-
Role of molecular chaperones and TPR-domain proteins in the cytoplasmic transport of steroid receptors and their passage through the nuclear pore.Nucleus. 2010 Jul-Aug;1(4):299-308. doi: 10.4161/nucl.1.4.11743. Nucleus. 2010. PMID: 21113270 Free PMC article.
-
Nucleocytoplasmic shuttling of the glucocorticoid receptor is influenced by tetratricopeptide repeat-containing proteins.J Cell Sci. 2020 Jun 16;133(12):jcs238873. doi: 10.1242/jcs.238873. J Cell Sci. 2020. PMID: 32467326
-
The hsp90-FKBP52 complex links the mineralocorticoid receptor to motor proteins and persists bound to the receptor in early nuclear events.Mol Cell Biol. 2010 Mar;30(5):1285-98. doi: 10.1128/MCB.01190-09. Epub 2009 Dec 28. Mol Cell Biol. 2010. PMID: 20038533 Free PMC article.
-
Chaperoning of glucocorticoid receptors.Handb Exp Pharmacol. 2006;(172):111-38. doi: 10.1007/3-540-29717-0_5. Handb Exp Pharmacol. 2006. PMID: 16610357 Review.
-
Therapeutic Targeting of the FKBP52 Co-Chaperone in Steroid Hormone Receptor-Regulated Physiology and Disease.Curr Mol Pharmacol. 2015;9(2):109-25. doi: 10.2174/1874467208666150519114115. Curr Mol Pharmacol. 2015. PMID: 25986565 Review.
Cited by
-
Nuclear distributions of NUP62 and NUP214 suggest architectural diversity and spatial patterning among nuclear pore complexes.PLoS One. 2012;7(4):e36137. doi: 10.1371/journal.pone.0036137. Epub 2012 Apr 27. PLoS One. 2012. PMID: 22558357 Free PMC article.
-
Evaluation of [11C]NMS-E973 as a PET tracer for in vivo visualisation of HSP90.Theranostics. 2019 Jan 1;9(2):554-572. doi: 10.7150/thno.27213. eCollection 2019. Theranostics. 2019. PMID: 30809293 Free PMC article.
-
SARS-CoV-2 ORF6 Disrupts Bidirectional Nucleocytoplasmic Transport through Interactions with Rae1 and Nup98.mBio. 2021 Apr 13;12(2):e00065-21. doi: 10.1128/mBio.00065-21. mBio. 2021. PMID: 33849972 Free PMC article.
-
Fluorescent protein-labeled glucocorticoid receptor alpha isoform trafficking in cultured human trabecular meshwork cells.Invest Ophthalmol Vis Sci. 2012 May 17;53(6):2938-50. doi: 10.1167/iovs.11-8331. Invest Ophthalmol Vis Sci. 2012. PMID: 22447868 Free PMC article.
-
SGT1 is required in PcINF1/SRC2-1 induced pepper defense response by interacting with SRC2-1.Sci Rep. 2016 Feb 22;6:21651. doi: 10.1038/srep21651. Sci Rep. 2016. PMID: 26898479 Free PMC article.
References
-
- Albermann, L., V. Shahin, Y. Ludwig, C. Schafer, H. Schillers, and H. Oberleithner. 2004. Evidence for importin alpha independent nuclear translocation of glucocorticoid receptors in Xenopus laevis oocytes. Cell. Physiol. Biochem. 14343-350. - PubMed
-
- Banerjee, A., S. Periyasamy, I. M. Wolf, T. D. Hinds, Jr., W. Yong, W. Shou, and E. R. Sanchez. 2008. Control of glucocorticoid and progesterone receptor subcellular localization by the ligand-binding domain is mediated by distinct interactions with tetratricopeptide repeat proteins. Biochemistry 4710471-10480. - PMC - PubMed
-
- Barsky, A., J. L. Gardy, R. E. Hancock, and T. Munzner. 2007. Cerebral: a Cytoscape plugin for layout of and interaction with biological networks using subcellular localization annotation. Bioinformatics 231040-1042. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
