Early low protein diet aggravates unbalance between antioxidant enzymes leading to islet dysfunction
- PMID: 19568427
- PMCID: PMC2699474
- DOI: 10.1371/journal.pone.0006110
Early low protein diet aggravates unbalance between antioxidant enzymes leading to islet dysfunction
Abstract
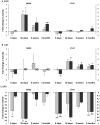
Background: Islets from adult rat possess weak antioxidant defense leading to unbalance between superoxide dismutase (SOD) and hydrogen peroxide-inactivating enzymatic activities, catalase (CAT) and glutathione peroxidase (GPX) rending them susceptible to oxidative stress. We have shown that this vulnerability is influenced by maternal diet during gestation and lactation.
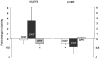
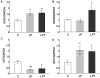
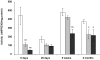
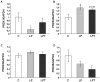
Methodology/principal findings: The present study investigated if low antioxidant activity in islets is already observed at birth and if maternal protein restriction influences the development of islet antioxidant defenses. Rats were fed a control diet (C group) or a low protein diet during gestation (LP) or until weaning (LPT), after which offspring received the control diet. We found that antioxidant enzymatic activities varied with age. At birth and after weaning, normal islets possessed an efficient GPX activity. However, the antioxidant capacity decreased thereafter increasing the potential vulnerability to oxidative stress. Maternal protein malnutrition changed the antioxidant enzymatic activities in islets of the progeny. At 3 months, SOD activity was increased in LP and LPT islets with no concomitant activation of CAT and GPX. This unbalance could lead to higher hydrogen peroxide production, which may concur to oxidative stress causing defective insulin gene expression due to modification of critical factors that modulate the insulin promoter. We found indeed that insulin mRNA level was reduced in both groups of malnourished offspring compared to controls. Analyzing the expression of such critical factors, we found that c-Myc expression was strongly increased in islets from both protein-restricted groups compared to controls.
Conclusion and significance: Modification in antioxidant activity by maternal low protein diet could predispose to pancreatic islet dysfunction later in life and provide new insights to define a molecular mechanism responsible for intrauterine programming of endocrine pancreas.
Conflict of interest statement
Figures
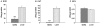
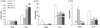

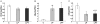
Similar articles
-
Reduced glucose-induced insulin secretion in low-protein-fed rats is associated with altered pancreatic islets redox status.J Cell Physiol. 2018 Jan;233(1):486-496. doi: 10.1002/jcp.25908. Epub 2017 May 3. J Cell Physiol. 2018. PMID: 28370189
-
Superoxide dismutase, catalase and glutathione peroxidase in the spontaneously hypertensive rat kidney: effect of antioxidant-rich diet.J Hypertens. 2004 Oct;22(10):2025-33. doi: 10.1097/00004872-200410000-00027. J Hypertens. 2004. PMID: 15361776
-
Altered pancreatic morphology in the offspring of pregnant rats given reduced dietary protein is time and gender specific.J Endocrinol. 2006 Oct;191(1):83-92. doi: 10.1677/joe.1.06754. J Endocrinol. 2006. PMID: 17065391
-
Differences in the expression of heat-shock proteins and antioxidant enzymes between human and rodent pancreatic islets: implications for the pathogenesis of insulin-dependent diabetes mellitus.Mol Med. 1995 Nov;1(7):806-20. Mol Med. 1995. PMID: 8612203 Free PMC article.
-
In utero oxidative stress epigenetically programs antioxidant defense capacity and adulthood diseases.Antioxid Redox Signal. 2012 Jul 15;17(2):237-53. doi: 10.1089/ars.2011.4372. Epub 2012 Jan 11. Antioxid Redox Signal. 2012. PMID: 22035055 Free PMC article. Review.
Cited by
-
Sustained elevation of NF-κB activity sensitizes offspring of maternal inflammation to hypertension via impairing PGC-1α recovery.Sci Rep. 2016 Sep 12;6:32642. doi: 10.1038/srep32642. Sci Rep. 2016. PMID: 27616627 Free PMC article.
-
Consequences of a compromised intrauterine environment on islet function.J Endocrinol. 2010 Jun;205(3):211-24. doi: 10.1677/JOE-09-0399. Epub 2010 Mar 11. J Endocrinol. 2010. PMID: 20223861 Free PMC article. Review.
-
Alteration of mitochondrial function in adult rat offspring of malnourished dams.World J Diabetes. 2011 Sep 15;2(9):149-57. doi: 10.4239/wjd.v2.i9.149. World J Diabetes. 2011. PMID: 21954419 Free PMC article.
-
Early Weaning Affects Liver Antioxidant Function in Piglets.Animals (Basel). 2021 Sep 13;11(9):2679. doi: 10.3390/ani11092679. Animals (Basel). 2021. PMID: 34573645 Free PMC article.
-
The Role of Cellular Stress in Intrauterine Growth Restriction and Postnatal Dysmetabolism.Int J Mol Sci. 2021 Jun 29;22(13):6986. doi: 10.3390/ijms22136986. Int J Mol Sci. 2021. PMID: 34209700 Free PMC article. Review.
References
-
- Lenzen S. Oxidative stress: the vulnerable beta-cell. Biochem Soc Trans. 2008;36:343–347. - PubMed
-
- Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes. 2003;52:1–8. - PubMed
-
- Robertson R, Zhou H, Zhang T, Harmon JS. Chronic oxidative stress as a mechanism for glucose toxicity of the beta cell in type 2 diabetes. Cell Biochem Biophys. 2007;48:139–146. - PubMed
-
- Cnop M, Welsh N, Jonas JC, Jorns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54(Suppl 2):S97–107. - PubMed
-
- Suarez-Pinzon WL, Szabo C, Rabinovitch A. Development of autoimmune diabetes in NOD mice is associated with the formation of peroxynitrite in pancreatic islet beta-cells. Diabetes. 1997;46:907–911. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

