Numb regulates post-endocytic trafficking and degradation of Notch1
- PMID: 19567869
- PMCID: PMC2785331
- DOI: 10.1074/jbc.M109.014845
Numb regulates post-endocytic trafficking and degradation of Notch1
Abstract
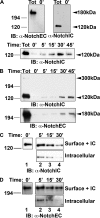
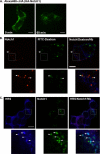
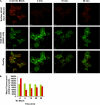
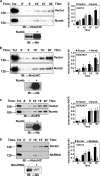
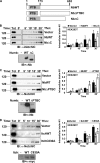
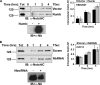
Notch is a transmembrane receptor that controls cell fate decisions during development and tissue homeostasis. Both activation and attenuation of the Notch signal are tightly regulated by endocytosis. The adaptor protein Numb acts as an inhibitor of Notch and is known to function within the intracellular trafficking pathways. However, a role for Numb in regulating Notch trafficking has not been defined. Here we show that mammalian Notch1 is constitutively internalized and trafficked to both recycling and late endosomal compartments, and we demonstrate that changes in Numb expression alter the dynamics of Notch1 trafficking. Overexpression of Numb promotes sorting of Notch1 through late endosomes for degradation, whereas depletion of Numb facilitates Notch1 recycling. Numb mutants that do not interact with the ubiquitin-protein isopeptide ligase, Itch, or that lack motifs important for interaction with endocytic proteins fail to promote Notch1 degradation. Our data suggest that Numb inhibits Notch1 activity by regulating post-endocytic sorting events that lead to Notch1 degradation.
Figures

Similar articles
-
Mammalian Numb protein antagonizes Notch by controlling postendocytic trafficking of the Notch ligand Delta-like 4.J Biol Chem. 2017 Dec 15;292(50):20628-20643. doi: 10.1074/jbc.M117.800946. Epub 2017 Oct 17. J Biol Chem. 2017. PMID: 29042443 Free PMC article.
-
Mammalian numb proteins promote Notch1 receptor ubiquitination and degradation of the Notch1 intracellular domain.J Biol Chem. 2003 Jun 20;278(25):23196-203. doi: 10.1074/jbc.M302827200. Epub 2003 Apr 7. J Biol Chem. 2003. PMID: 12682059
-
Numb regulates Notch1, but not Notch3, during myogenesis.Mech Dev. 2011 May-Jun;128(5-6):247-57. doi: 10.1016/j.mod.2011.02.002. Epub 2011 Feb 26. Mech Dev. 2011. PMID: 21356309
-
NUMB-ing down cancer by more than just a NOTCH.Biochim Biophys Acta. 2011 Jan;1815(1):26-43. doi: 10.1016/j.bbcan.2010.10.001. Epub 2010 Oct 16. Biochim Biophys Acta. 2011. PMID: 20940030 Review.
-
Complementary roles of the neuron-enriched endosomal proteins NEEP21 and calcyon in neuronal vesicle trafficking.J Neurochem. 2015 Jan;132(1):20-31. doi: 10.1111/jnc.12989. J Neurochem. 2015. PMID: 25376768 Review.
Cited by
-
Regulation of Numb isoform expression by activated ERK signaling.Oncogene. 2016 Sep 29;35(39):5202-13. doi: 10.1038/onc.2016.69. Epub 2016 Apr 4. Oncogene. 2016. PMID: 27041567
-
Non-canonical Notch signaling: emerging role and mechanism.Trends Cell Biol. 2012 May;22(5):257-65. doi: 10.1016/j.tcb.2012.02.003. Epub 2012 Mar 5. Trends Cell Biol. 2012. PMID: 22397947 Free PMC article. Review.
-
Notch signaling determines cell-fate specification of the two main types of vomeronasal neurons of rodents.Development. 2022 Jul 1;149(13):dev200448. doi: 10.1242/dev.200448. Epub 2022 Jul 4. Development. 2022. PMID: 35781337 Free PMC article.
-
A fluorescent tagging approach in Drosophila reveals late endosomal trafficking of Notch and Sanpodo.J Cell Biol. 2014 Nov 10;207(3):351-63. doi: 10.1083/jcb.201407071. Epub 2014 Nov 3. J Cell Biol. 2014. PMID: 25365996 Free PMC article.
-
The Spatiotemporal Expression of Notch1 and Numb and Their Functional Interaction during Cardiac Morphogenesis.Cells. 2021 Aug 25;10(9):2192. doi: 10.3390/cells10092192. Cells. 2021. PMID: 34571841 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

