Infectious tolerance via the consumption of essential amino acids and mTOR signaling
- PMID: 19567830
- PMCID: PMC2704109
- DOI: 10.1073/pnas.0903919106
Infectious tolerance via the consumption of essential amino acids and mTOR signaling
Abstract
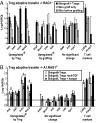
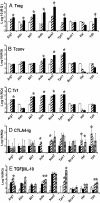
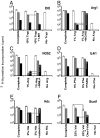
Infectious tolerance describes the process of CD4(+) regulatory T cells (Tregs) converting naïve T cells to become additional Tregs. We show that antigen-specific Tregs induce, within skin grafts and dendritic cells, the expression of enzymes that consume at least 5 different essential amino acids (EAAs). T cells fail to proliferate in response to antigen when any 1, or more, of these EAAs are limiting, which is associated with a reduced mammalian target of rapamycin (mTOR) signaling. Inhibition of the mTOR pathway by limiting EAAs, or by specific inhibitors, induces the Treg-specific transcription factor forkhead box P3, which depends on both T cell receptor activation and synergy with TGF-beta.
Conflict of interest statement
Conflict of interest statement: S.P.C. and H.W. are shareholders in TolerRx Inc. and receive royalties for CAMPATH antibody sales. S.P.C. is a shareholder and adviser to BioAnaLab Ltd. A.L.M. has intellectual property interests in the therapeutic use of IDO and IDO inhibitors and receives consulting income from NewLink Genetics Inc.
Figures
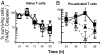
Similar articles
-
Synergy between rapamycin and FLT3 ligand enhances plasmacytoid dendritic cell-dependent induction of CD4+CD25+FoxP3+ Treg.Blood. 2015 May 7;125(19):2937-47. doi: 10.1182/blood-2014-09-599266. Epub 2015 Apr 1. Blood. 2015. PMID: 25833958 Free PMC article.
-
Induction of granzyme B expression in T-cell receptor/CD28-stimulated human regulatory T cells is suppressed by inhibitors of the PI3K-mTOR pathway.BMC Immunol. 2009 Nov 22;10:59. doi: 10.1186/1471-2172-10-59. BMC Immunol. 2009. PMID: 19930596 Free PMC article.
-
Interplay between mTOR and STAT5 signaling modulates the balance between regulatory and effective T cells.Immunobiology. 2015 Apr;220(4):510-7. doi: 10.1016/j.imbio.2014.10.020. Epub 2014 Oct 31. Immunobiology. 2015. PMID: 25468562
-
Immunoregulatory functions of mTOR inhibition.Nat Rev Immunol. 2009 May;9(5):324-37. doi: 10.1038/nri2546. Nat Rev Immunol. 2009. PMID: 19390566 Free PMC article. Review.
-
The mTOR pathway and integrating immune regulation.Immunology. 2013 Dec;140(4):391-8. doi: 10.1111/imm.12162. Immunology. 2013. PMID: 23952610 Free PMC article. Review.
Cited by
-
mTOR and lymphocyte metabolism.Curr Opin Immunol. 2013 Jun;25(3):347-55. doi: 10.1016/j.coi.2013.05.002. Epub 2013 May 28. Curr Opin Immunol. 2013. PMID: 23722114 Free PMC article. Review.
-
Regulation of immune responses by mTOR.Annu Rev Immunol. 2012;30:39-68. doi: 10.1146/annurev-immunol-020711-075024. Epub 2011 Nov 29. Annu Rev Immunol. 2012. PMID: 22136167 Free PMC article. Review.
-
Therapeutic opportunities for manipulating T(Reg) cells in autoimmunity and cancer.Nat Rev Drug Discov. 2013 Jan;12(1):51-63. doi: 10.1038/nrd3683. Nat Rev Drug Discov. 2013. PMID: 23274471 Review.
-
Immunomodulatory effects of deacetylase inhibitors: therapeutic targeting of FOXP3+ regulatory T cells.Nat Rev Drug Discov. 2009 Dec;8(12):969-81. doi: 10.1038/nrd3031. Epub 2009 Oct 26. Nat Rev Drug Discov. 2009. PMID: 19855427 Free PMC article. Review.
-
Enhancement of antigen-specific Treg vaccination in vivo.Proc Natl Acad Sci U S A. 2010 Sep 14;107(37):16246-51. doi: 10.1073/pnas.1007422107. Epub 2010 Aug 30. Proc Natl Acad Sci U S A. 2010. PMID: 20805478 Free PMC article.
References
-
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. - PubMed
-
- Wildin RS, et al. X-linked neonatal diabetes mellitus, enteropathy, and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. - PubMed
-
- Cobbold SP, et al. Immune privilege induced by regulatory T cells in transplantation tolerance. Immunol Rev. 2006;213:239–255. - PubMed
-
- Cobbold SP, et al. Induction of foxP3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J Immunol. 2004;172:6003–6010. - PubMed
-
- Qin S, et al. “Infectious” transplantation tolerance. Science. 1993;259:974–977. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

