A critical analysis of current in vitro and in vivo angiogenesis assays
- PMID: 19563606
- PMCID: PMC2697546
- DOI: 10.1111/j.1365-2613.2008.00633.x
A critical analysis of current in vitro and in vivo angiogenesis assays
Abstract
The study of angiogenesis has grown exponentially over the past 40 years with the recognition that angiogenesis is essential for numerous pathologies and, more recently, with the advent of successful drugs to inhibit angiogenesis in tumours. The main problem with angiogenesis research remains the choice of appropriate assays to evaluate the efficacy of potential new drugs and to identify potential targets within the angiogenic process. This selection is made more complex by the recognition that heterogeneity occurs, not only within the endothelial cells themselves, but also within the specific microenvironment to be studied. Thus, it is essential to choose the assay conditions and cell types that most closely resemble the angiogenic disease being studied. This is especially important when aiming to translate data from in vitro to in vivo and from preclinical to the clinic. Here we critically review and highlight recent advances in the principle assays in common use including those for endothelial cell proliferation, migration, differentiation and co-culture with fibroblasts and mural cells in vitro, vessel outgrowth from organ cultures and in vivo assays such as chick chorioallantoic membrane (CAM), zebrafish, sponge implantation, corneal, dorsal air sac, chamber and tumour angiogenesis models. Finally, we briefly discuss the direction likely to be taken in future studies, which include the use of increasingly sophisticated imaging analysis systems for data acquisition.
Figures




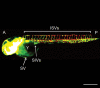

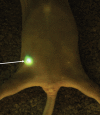
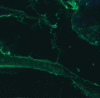
Similar articles
-
Current methods for assaying angiogenesis in vitro and in vivo.Int J Exp Pathol. 2004 Oct;85(5):233-48. doi: 10.1111/j.0959-9673.2004.00396.x. Int J Exp Pathol. 2004. PMID: 15379956 Free PMC article. Review.
-
In vitro assays of angiogenesis for assessment of angiogenic and anti-angiogenic agents.Microvasc Res. 2007 Sep-Nov;74(2-3):172-83. doi: 10.1016/j.mvr.2007.05.006. Epub 2007 Jun 6. Microvasc Res. 2007. PMID: 17631914 Free PMC article. Review.
-
Low-dose metronomic chemotherapy with cisplatin: can it suppress angiogenesis in H22 hepatocarcinoma cells?Int J Exp Pathol. 2010 Feb;91(1):10-6. doi: 10.1111/j.1365-2613.2009.00684.x. Int J Exp Pathol. 2010. PMID: 20096070 Free PMC article.
-
Robust and Scalable Angiogenesis Assay of Perfused 3D Human iPSC-Derived Endothelium for Anti-Angiogenic Drug Screening.Int J Mol Sci. 2020 Jul 7;21(13):4804. doi: 10.3390/ijms21134804. Int J Mol Sci. 2020. PMID: 32645937 Free PMC article.
-
Use of a monoclonal antibody specific for activated endothelial cells to quantitate angiogenesis in vivo in zebrafish after drug treatment.Angiogenesis. 2004;7(3):243-53. doi: 10.1007/s10456-004-4181-7. Angiogenesis. 2004. PMID: 15609079
Cited by
-
The angiogenic potential of CD271+ human adipose tissue-derived mesenchymal stem cells.Stem Cell Res Ther. 2021 Mar 2;12(1):160. doi: 10.1186/s13287-021-02177-0. Stem Cell Res Ther. 2021. PMID: 33653407 Free PMC article.
-
The Therapeutic Effects of Human Mesenchymal Stem Cells Primed with Sphingosine-1 Phosphate on Pulmonary Artery Hypertension.Stem Cells Dev. 2015 Jul 15;24(14):1658-71. doi: 10.1089/scd.2014.0496. Epub 2015 Apr 9. Stem Cells Dev. 2015. PMID: 25761906 Free PMC article.
-
The Signature Amino Acid Residue Serine 31 of HIV-1C Tat Potentiates an Activated Phenotype in Endothelial Cells.Front Immunol. 2020 Sep 25;11:529614. doi: 10.3389/fimmu.2020.529614. eCollection 2020. Front Immunol. 2020. PMID: 33101270 Free PMC article.
-
Engineering more than a cell: vascularization strategies in tissue engineering.Curr Opin Biotechnol. 2010 Oct;21(5):704-9. doi: 10.1016/j.copbio.2010.06.005. Epub 2010 Jul 16. Curr Opin Biotechnol. 2010. PMID: 20638268 Free PMC article. Review.
-
The Use of Live Cell Imaging and Automated Image Analysis to Assist With Determining Optimal Parameters for Angiogenic Assay in vitro.Front Cell Dev Biol. 2019 Apr 10;7:45. doi: 10.3389/fcell.2019.00045. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31024908 Free PMC article.
References
-
- Ades EW, Candal FJ, Swerlick RA, et al. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J. Invest. Dermatol. 1992;99:683–690. - PubMed
-
- Aird WC. Endothelial cell heterogeneity. Crit. Care Med. 2003;31:S221–S230. - PubMed
-
- Akimoto S, Mitsumata M, Sasaguri T, Yoshida Y. Laminar shear stress inhibits vascular endothelial cell proliferation by inducing cyclin-dependent kinase inhibitor p21(Sdi1/Cip1/Waf1) Circ. Res. 2000;86:185–190. - PubMed
-
- Alavi A, Hood JD, Frausto R, Stupack DG, Cheresh DA. Role of Raf in vascular protection from distinct apoptotic stimuli. Science. 2003;301:94–96. - PubMed
-
- Albini A, Benelli R, Noonan DM, Brigati C. The “chemoinvasion assay”: a tool to study tumor and endothelial cell invasion of basement membranes. Int. J. Dev. Biol. 2004;48:563–571. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

