A quest for the mechanism regulating global planar cell polarity of tissues
- PMID: 19560358
- PMCID: PMC3501338
- DOI: 10.1016/j.tcb.2009.04.003
A quest for the mechanism regulating global planar cell polarity of tissues
Abstract
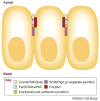
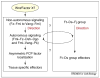
Most epithelial cells, besides their ubiquitous apical-basal polarity, are polarized within the plane of the epithelium, which is called planar cell polarity (PCP). Using Drosophila as a model, meaningful progress has been made in the identification of key PCP factors and the dissection of their intracellular molecular interactions. The long-range, global aspects of coordinated polarization and the overlying regulatory mechanisms that create the initial polarity direction have, however, remained elusive. Several recent publications have outlined potential mechanisms of how the global regulation of PCP might be controlled and how the distinct core factor groups might interact via frizzled, Van Gogh or flamingo. This review focuses on these exciting features and attempts to provide an integrated picture of these recent and novel insights.
Figures


Similar articles
-
Planar cell polarity signaling: coordination of cellular orientation across tissues.Wiley Interdiscip Rev Dev Biol. 2012 Jul-Aug;1(4):479-99. doi: 10.1002/wdev.32. Wiley Interdiscip Rev Dev Biol. 2012. PMID: 23066429 Free PMC article. Review.
-
Asymmetric homotypic interactions of the atypical cadherin flamingo mediate intercellular polarity signaling.Cell. 2008 Jun 13;133(6):1093-105. doi: 10.1016/j.cell.2008.04.048. Cell. 2008. PMID: 18555784 Free PMC article.
-
Diego interacts with Prickle and Strabismus/Van Gogh to localize planar cell polarity complexes.Development. 2004 Sep;131(18):4467-76. doi: 10.1242/dev.01317. Epub 2004 Aug 11. Development. 2004. PMID: 15306567
-
Wnt-Frizzled/planar cell polarity signaling: cellular orientation by facing the wind (Wnt).Annu Rev Cell Dev Biol. 2015;31:623-46. doi: 10.1146/annurev-cellbio-100814-125315. Annu Rev Cell Dev Biol. 2015. PMID: 26566118 Free PMC article. Review.
-
The apical/basal-polarity determinant Scribble cooperates with the PCP core factor Stbm/Vang and functions as one of its effectors.Dev Biol. 2009 Sep 1;333(1):67-77. doi: 10.1016/j.ydbio.2009.06.024. Epub 2009 Jun 27. Dev Biol. 2009. PMID: 19563796 Free PMC article.
Cited by
-
Crumbs protein homolog 3 (CRB3) expression is associated with oestrogen and progesterone receptor positivity in breast cancer.Clin Exp Pharmacol Physiol. 2019 Sep;46(9):837-844. doi: 10.1111/1440-1681.13104. Epub 2019 Jun 10. Clin Exp Pharmacol Physiol. 2019. PMID: 31087799 Free PMC article.
-
Translating cell polarity into tissue elongation.Semin Cell Dev Biol. 2011 Oct;22(8):858-64. doi: 10.1016/j.semcdb.2011.09.013. Epub 2011 Oct 1. Semin Cell Dev Biol. 2011. PMID: 21983030 Free PMC article. Review.
-
Planar cell polarity signaling: coordination of cellular orientation across tissues.Wiley Interdiscip Rev Dev Biol. 2012 Jul-Aug;1(4):479-99. doi: 10.1002/wdev.32. Wiley Interdiscip Rev Dev Biol. 2012. PMID: 23066429 Free PMC article. Review.
-
Nuclear signaling from cadherin adhesion complexes.Curr Top Dev Biol. 2015;112:129-96. doi: 10.1016/bs.ctdb.2014.11.018. Epub 2015 Feb 12. Curr Top Dev Biol. 2015. PMID: 25733140 Free PMC article. Review.
-
Co-regulation of polar mRNA transport and lifespan in budding yeast Saccharomyces cerevisiae.Cell Cycle. 2012 Nov 15;11(22):4275-80. doi: 10.4161/cc.22659. Epub 2012 Oct 30. Cell Cycle. 2012. PMID: 23111244 Free PMC article.
References
-
- Adler PN. Planar signaling and morphogenesis in Drosophila. Dev Cell. 2002;2:525–535. - PubMed
-
- Seifert JR, Mlodzik M. Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat Rev. 2007;8:126–138. - PubMed
-
- Strutt D. Frizzled signalling and cell polarisation in Drosophila and vertebrates. Development. 2003;130:4501–4513. - PubMed
-
- Karner C, et al. Planar cell polarity and vertebrate organogenesis. Semin Cell Dev Biol. 2006;17:194–203. - PubMed
-
- Wang Y, Nathans J. Tissue/planar cell polarity in vertebrates: new insights and new questions. Development. 2007;134:647–658. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

