A novel flow cytometric high throughput assay for a systematic study on molecular mechanisms underlying T cell receptor-mediated integrin activation
- PMID: 19557182
- PMCID: PMC2698288
- DOI: 10.1371/journal.pone.0006044
A novel flow cytometric high throughput assay for a systematic study on molecular mechanisms underlying T cell receptor-mediated integrin activation
Abstract
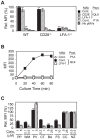
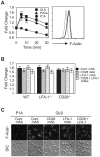
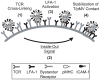
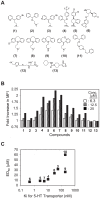
Lymphocyte function-associated antigen 1 (LFA-1), a member of beta2-integrin family, exerts multiple roles in host T cell immunity and has been identified as a useful drug-development target for inflammatory and autoimmune diseases. Applying the findings that primary resting T cells absorb nanometric membrane vesicles derived from antigen presenting cells (APC) via dual receptor/ligand interactions of T cell receptor (TCR) with cognate peptide-major histocompatibility complex (MHC) complex (pMHC) and LFA-1 with its ligand, intercellular adhesion molecule-1 (ICAM-1), and that signaling cascades triggered by TCR/pMHC interaction take a part in the vesicle-absorption, we established a cell-based high throughput assay for systematic investigation, via isolation of small molecules modulating the level of vesicle-absorption, of molecular mechanisms underlying the T cell absorption of APC-derived vesicles, i.e., structural basis of TCR/pMHC and LFA-1/ICAM-1 interactions and TCR-mediated LFA-1 activation. As primary T cells along with physiological ligands expressed in biological membrane are used and also individual cells in assay samples are analyzed by flow cytometry, results obtained using the assay system hold superior physiological and therapeutic relevance as well as statistical precision.
Conflict of interest statement
Figures

Similar articles
-
Acute inhibition of selected membrane-proximal mouse T cell receptor signaling by mitochondrial antagonists.PLoS One. 2009 Nov 10;4(11):e7738. doi: 10.1371/journal.pone.0007738. PLoS One. 2009. PMID: 19901985 Free PMC article.
-
Cutting edge: LFA-1 integrin-dependent T cell adhesion is regulated by both ag specificity and sensitivity.J Immunol. 2004 Aug 15;173(4):2222-6. doi: 10.4049/jimmunol.173.4.2222. J Immunol. 2004. PMID: 15294931
-
The dependence for leukocyte function-associated antigen-1/ICAM-1 interactions in T cell activation cannot be overcome by expression of high density TCR ligand.J Immunol. 1999 Apr 15;162(8):4399-405. J Immunol. 1999. PMID: 10201975
-
T Cell Activation Pathways: B7, LFA-3, and ICAM-1 Shape Unique T Cell Profiles.Crit Rev Immunol. 2017;37(2-6):463-481. doi: 10.1615/CritRevImmunol.v37.i2-6.130. Crit Rev Immunol. 2017. PMID: 29773030 Review.
-
Inhibition of LFA-1/ICAM-1 and VLA-4/VCAM-1 as a therapeutic approach to inflammation and autoimmune diseases.Med Res Rev. 2002 Mar;22(2):146-67. doi: 10.1002/med.10001. Med Res Rev. 2002. PMID: 11857637 Review.
Cited by
-
LFA-1-dependent Ca2+ entry following suboptimal T cell receptor triggering proceeds without mobilization of intracellular Ca2+.J Biol Chem. 2009 Aug 14;284(33):22149-22154. doi: 10.1074/jbc.M109.000752. Epub 2009 Jun 19. J Biol Chem. 2009. PMID: 19542227 Free PMC article.
-
Acute inhibition of selected membrane-proximal mouse T cell receptor signaling by mitochondrial antagonists.PLoS One. 2009 Nov 10;4(11):e7738. doi: 10.1371/journal.pone.0007738. PLoS One. 2009. PMID: 19901985 Free PMC article.
-
Receptor-mediated T cell absorption of antigen presenting cell-derived molecules.Front Biosci (Landmark Ed). 2011 Jan 1;16(2):411-21. doi: 10.2741/3695. Front Biosci (Landmark Ed). 2011. PMID: 21196178 Free PMC article. Review.
-
Flow Cytometry: Impact on Early Drug Discovery.J Biomol Screen. 2015 Jul;20(6):689-707. doi: 10.1177/1087057115578273. Epub 2015 Mar 24. J Biomol Screen. 2015. PMID: 25805180 Free PMC article. Review.
-
Flow cytometry enables a high-throughput homogeneous fluorescent antibody-binding assay for cytotoxic T cell lytic granule exocytosis.J Biomol Screen. 2013 Apr;18(4):420-9. doi: 10.1177/1087057112466697. Epub 2012 Nov 15. J Biomol Screen. 2013. PMID: 23160568 Free PMC article.
References
-
- Pribila JT, Quale AC, Mueller KL, Shimizu Y. Integrins and T cell-mediated immunity. Annu Rev Immunol. 2004;22:157–180. - PubMed
-
- Kuhn JR, Poenie M. Dynamic polarization of the microtubule cytoskeleton during CTL-mediated killing. Immunity. 2002;16:111–121. - PubMed
-
- Mousa SA. Cell adhesion molecules: potential therapeutic and diagnostic implications. Methods Mol Med. 2004;93:157–174. - PubMed
-
- Nishibori M, Takahashi HK, Mori S. The regulation of ICAM-1 and LFA-1 interaction by autacoids and statins: a novel strategy for controlling inflammation and immune responses. J Pharmacol Sci. 2003;92:7–12. - PubMed
-
- Hogg N, Henderson R, Leitinger B, McDowall A, Porter J, et al. Mechanisms contributing to the activity of integrins on leukocytes. Immunol Rev. 2002;186:164–171. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

