BRG1 increases transcription of proinflammatory genes in renal ischemia
- PMID: 19556365
- PMCID: PMC2723991
- DOI: 10.1681/ASN.2009010118
BRG1 increases transcription of proinflammatory genes in renal ischemia
Abstract
Acute kidney injury stimulates renal production of inflammatory mediators, including TNF-alpha and monocyte chemoattractant protein 1 (MCP-1). These responses reflect, in part, injury-induced transcription of proinflammatory genes by proximal tubule cells. Because of the compact structure of chromatin, a series of events at specified loci remodel chromatin to provide access for transcription factors and RNA polymerase II (Pol II). Here, we examined the role of Brahma-related gene-1 (BRG1), a chromatin remodeling enzyme, in the transcription of TNF-alpha and MCP-1 in response to renal ischemia. Two hours after renal ischemic injury in mice, renal TNF-alpha and MCP-1 mRNA increased and remained elevated for at least 1 wk. Matrix chromatin immunoprecipitation assays revealed sustained increases in Pol II at these genes, suggesting that the elevated mRNA levels were, at least in part, transcriptionally mediated. The profile of BGR1 binding to the genes encoding TNF-alpha and MCP-1 resembled Pol II recruitment. Knockdown of BRG1 by small interfering RNA blocked an ATP depletion-induced increase in TNF-alpha and MCP-1 transcription in a human proximal tubule cell line; this effect was associated with decreased recruitment of BRG1 and Pol II to these genes. In conclusion, BRG1 promotes increased transcription of TNF-alpha and MCP-1 by the proximal tubule in response to renal ischemia.
Figures
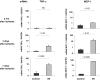
 ). The contralateral kidneys, not subjected to I/R, served as time-matched controls (■). The mRNA levels of TNF-α and MCP-1 were assessed by competitive PCR and are expressed as a ratio to the simultaneously obtained glyceraldehyde-3-phosphate dehydrogenase (GAPDH) transcript. Data are means ± 1 SD (n = 4 mice).
). The contralateral kidneys, not subjected to I/R, served as time-matched controls (■). The mRNA levels of TNF-α and MCP-1 were assessed by competitive PCR and are expressed as a ratio to the simultaneously obtained glyceraldehyde-3-phosphate dehydrogenase (GAPDH) transcript. Data are means ± 1 SD (n = 4 mice).
 ). (A) Levels at the TNF-α first (exon 1) and last (exon 4) exons were assessed using matrix ChIP assays., (B) Levels measured in an intergenic region 5 kb downstream of the end of the TNF-α gene served as a control. Data are percentage of input DNA, mean ± 1 SD (n = 3 mice).
). (A) Levels at the TNF-α first (exon 1) and last (exon 4) exons were assessed using matrix ChIP assays., (B) Levels measured in an intergenic region 5 kb downstream of the end of the TNF-α gene served as a control. Data are percentage of input DNA, mean ± 1 SD (n = 3 mice).
 ). (A) Levels at the MCP-1 first (exon 1) and last (exon 3) exons were assessed using matrix ChIP assays. (B) Levels measured in an intergenic region 5 kb downstream of the end of the MCP-1 gene served as a control. Data are percentage of input DNA, mean ± 1 SD (n = 3 mice).
). (A) Levels at the MCP-1 first (exon 1) and last (exon 3) exons were assessed using matrix ChIP assays. (B) Levels measured in an intergenic region 5 kb downstream of the end of the MCP-1 gene served as a control. Data are percentage of input DNA, mean ± 1 SD (n = 3 mice).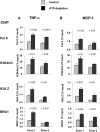
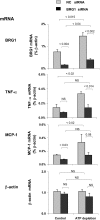
 ), HK-2 cells were treated either without (Control) or with (ATP depletion AA + DOG). Total RNA was extracted and reverse-transcribed, and transcript levels were assessed by real-time PCR done in triplicate using specific primers. mRNA levels are expressed as percentage of β-actin transcript. Data are means ± 1 SD (n = 3 experiments).
), HK-2 cells were treated either without (Control) or with (ATP depletion AA + DOG). Total RNA was extracted and reverse-transcribed, and transcript levels were assessed by real-time PCR done in triplicate using specific primers. mRNA levels are expressed as percentage of β-actin transcript. Data are means ± 1 SD (n = 3 experiments).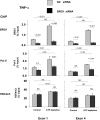
 ). After transfection, HK-2 cells were treated either without (Control) or with (ATP depletion) AA + DOG; chromatin was extracted and sheared. Density at the TNF-α first (exon 1) and last (exon 4) exons were assessed using matrix ChIP assay. Data are percentage of input DNA, mean ± 1 SD (n = 3 experiments).
). After transfection, HK-2 cells were treated either without (Control) or with (ATP depletion) AA + DOG; chromatin was extracted and sheared. Density at the TNF-α first (exon 1) and last (exon 4) exons were assessed using matrix ChIP assay. Data are percentage of input DNA, mean ± 1 SD (n = 3 experiments).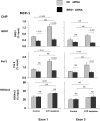
 ). After transfection, HK-2 cells were treated either without (Control) or with (ATP depletion) AA + DOG; chromatin was extracted and sheared. Density at the MCP-1 first (exon 1) and last (exon 3) exons were assessed using matrix ChIP assay. Data are percentage of input DNA, mean ± 1 SD (n = 3 experiments).
). After transfection, HK-2 cells were treated either without (Control) or with (ATP depletion) AA + DOG; chromatin was extracted and sheared. Density at the MCP-1 first (exon 1) and last (exon 3) exons were assessed using matrix ChIP assay. Data are percentage of input DNA, mean ± 1 SD (n = 3 experiments).Similar articles
-
Renal ischemia-reperfusion injury upregulates histone-modifying enzyme systems and alters histone expression at proinflammatory/profibrotic genes.Am J Physiol Renal Physiol. 2009 May;296(5):F1032-41. doi: 10.1152/ajprenal.00061.2009. Epub 2009 Mar 4. Am J Physiol Renal Physiol. 2009. PMID: 19261745 Free PMC article.
-
BRG1 regulates endothelial-derived IL-33 to promote ischemia-reperfusion induced renal injury and fibrosis in mice.Biochim Biophys Acta Mol Basis Dis. 2019 Sep 1;1865(9):2551-2561. doi: 10.1016/j.bbadis.2019.06.015. Epub 2019 Jun 19. Biochim Biophys Acta Mol Basis Dis. 2019. PMID: 31228616
-
Uremia impacts renal inflammatory cytokine gene expression in the setting of experimental acute kidney injury.Am J Physiol Renal Physiol. 2009 Oct;297(4):F961-70. doi: 10.1152/ajprenal.00381.2009. Epub 2009 Aug 5. Am J Physiol Renal Physiol. 2009. PMID: 19656911 Free PMC article.
-
Progressive histone alterations and proinflammatory gene activation: consequences of heme protein/iron-mediated proximal tubule injury.Am J Physiol Renal Physiol. 2010 Mar;298(3):F827-37. doi: 10.1152/ajprenal.00683.2009. Epub 2009 Dec 23. Am J Physiol Renal Physiol. 2010. PMID: 20032114 Free PMC article.
-
Endotoxin mediates recruitment of RNA polymerase II to target genes in acute renal failure.J Am Soc Nephrol. 2008 Jul;19(7):1321-30. doi: 10.1681/ASN.2007121368. Epub 2008 Apr 16. J Am Soc Nephrol. 2008. PMID: 18417719 Free PMC article.
Cited by
-
Epigenetic Modification Mechanisms Involved in Inflammation and Fibrosis in Renal Pathology.Mediators Inflamm. 2018 Dec 13;2018:2931049. doi: 10.1155/2018/2931049. eCollection 2018. Mediators Inflamm. 2018. PMID: 30647531 Free PMC article. Review.
-
MCP-1 gene activation marks acute kidney injury.J Am Soc Nephrol. 2011 Jan;22(1):165-75. doi: 10.1681/ASN.2010060641. Epub 2010 Nov 11. J Am Soc Nephrol. 2011. PMID: 21071523 Free PMC article.
-
Endothelial Progenitor Cells Modulate Inflammation-Associated Stroke Vasculome.Stem Cell Rev Rep. 2019 Apr;15(2):256-275. doi: 10.1007/s12015-019-9873-x. Stem Cell Rev Rep. 2019. PMID: 30739275 Free PMC article.
-
Nascent proteomes of ischemic-injured and ischemic-tolerant neuronal cells.Int J Comput Biol Drug Des. 2011;4(1):40-55. doi: 10.1504/IJCBDD.2011.038656. Epub 2011 Feb 17. Int J Comput Biol Drug Des. 2011. PMID: 21330693 Free PMC article.
-
Acute kidney injury and chronic kidney disease: From the laboratory to the clinic.Nephrol Ther. 2016 Apr;12 Suppl 1(Suppl 1):S41-8. doi: 10.1016/j.nephro.2016.02.005. Epub 2016 Mar 10. Nephrol Ther. 2016. PMID: 26972097 Free PMC article. Review.
References
-
- Zager RA, Johnson AC, Lund S: ‘Endotoxin tolerance’: TNF-alpha hyper-reactivity and tubular cytoresistance in a renal cholesterol loading state. Kidney Int 71: 496–503, 2007 - PubMed
-
- Zager RA, Johnson AC, Hanson SY, Lund S: Acute nephrotoxic and obstructive injury primes the kidney to endotoxin-driven cytokine/chemokine production. Kidney Int 69: 1181–1188, 2006 - PubMed
-
- Zager RA, Johnson AC, Lund S, Hanson S: Acute renal failure: Determinants and characteristics of the injury-induced hyperinflammatory response. Am J Physiol Renal Physiol 291: F546–F556, 2006 - PubMed
-
- Ramesh G, Zhang B, Uematsu S, Akira S, Reeves WB: Endotoxin and cisplatin synergistically induce renal dysfunction and cytokine production in mice. Am J Physiol Renal Physiol 293: F325–F332, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

