The glial nature of embryonic and adult neural stem cells
- PMID: 19555289
- PMCID: PMC3086722
- DOI: 10.1146/annurev.neuro.051508.135600
The glial nature of embryonic and adult neural stem cells
Abstract
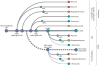
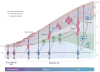
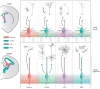
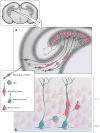
Glial cells were long considered end products of neural differentiation, specialized supportive cells with an origin very different from that of neurons. New studies have shown that some glial cells--radial glia (RG) in development and specific subpopulations of astrocytes in adult mammals--function as primary progenitors or neural stem cells (NSCs). This is a fundamental departure from classical views separating neuronal and glial lineages early in development. Direct visualization of the behavior of NSCs and lineage-tracing studies reveal how neuronal lineages emerge. In development and in the adult brain, many neurons and glial cells are not the direct progeny of NSCs, but instead originate from transit amplifying, or intermediate, progenitor cells (IPCs). Within NSCs and IPCs, genetic programs unfold for generating the extraordinary diversity of cell types in the central nervous system. The timing in development and location of NSCs, a property tightly linked to their neuroepithelial origin, appear to be the key determinants of the types of neurons generated. Identification of NSCs and IPCs is critical to understand brain development and adult neurogenesis and to develop new strategies for brain repair.
Figures


Similar articles
-
Neural stem cells in mammalian development.Curr Opin Cell Biol. 2006 Dec;18(6):704-9. doi: 10.1016/j.ceb.2006.09.008. Epub 2006 Oct 12. Curr Opin Cell Biol. 2006. PMID: 17046226 Review.
-
Developmental expression of fibroblast growth factor (FGF) receptors in neural stem cell progeny. Modulation of neuronal and glial lineages by basic FGF treatment.Neurol Res. 2001 Sep;23(6):612-21. doi: 10.1179/016164101101199090. Neurol Res. 2001. PMID: 11547930
-
The glial identity of neural stem cells.Nat Neurosci. 2003 Nov;6(11):1127-34. doi: 10.1038/nn1144. Epub 2003 Oct 28. Nat Neurosci. 2003. PMID: 14583753 Review.
-
Radial glia diversity: a matter of cell fate.Glia. 2003 Jul;43(1):37-43. doi: 10.1002/glia.10250. Glia. 2003. PMID: 12761864 Review.
-
The cell biology of neurogenesis.Nat Rev Mol Cell Biol. 2005 Oct;6(10):777-88. doi: 10.1038/nrm1739. Nat Rev Mol Cell Biol. 2005. PMID: 16314867 Review.
Cited by
-
Neural stem cells in the adult human brain.Biol Biomed Rep. 2012;2(1):59-69. Biol Biomed Rep. 2012. PMID: 23181200 Free PMC article.
-
Protective astrogenesis from the SVZ niche after injury is controlled by Notch modulator Thbs4.Nature. 2013 May 16;497(7449):369-73. doi: 10.1038/nature12069. Epub 2013 Apr 24. Nature. 2013. PMID: 23615612 Free PMC article.
-
Stem cells for spinal cord injury: Strategies to inform differentiation and transplantation.Biotechnol Bioeng. 2017 Feb;114(2):245-259. doi: 10.1002/bit.26074. Epub 2016 Sep 21. Biotechnol Bioeng. 2017. PMID: 27531038 Free PMC article. Review.
-
A global transcriptome analysis reveals molecular hallmarks of neural stem cell death, survival, and differentiation in response to partial FGF-2 and EGF deprivation.PLoS One. 2013;8(1):e53594. doi: 10.1371/journal.pone.0053594. Epub 2013 Jan 7. PLoS One. 2013. PMID: 23308259 Free PMC article.
-
The frontier of RNA metamorphosis and ribosome signature in neocortical development.Int J Dev Neurosci. 2016 Dec;55:131-139. doi: 10.1016/j.ijdevneu.2016.02.003. Epub 2016 May 27. Int J Dev Neurosci. 2016. PMID: 27241046 Free PMC article. Review.
References
-
- Aaku-Saraste E, Oback B, Hellwig A, Huttner WB. Neuroepithelial cells downregulate their plasma membrane polarity prior to neural tube closure and neurogenesis. Mech Dev. 1997;69:71–81. - PubMed
-
- Aguirre A, Dupree JL, Mangin JM, Gallo V. A functional role for EGFR signaling in myelination and remyelination. Nat Neurosci. 2007;10:990–1002. - PubMed
-
- Altman J, Bayer SA. Horizontal compartmentation in the germinal matrices and intermediate zone of the embryonic rat cerebral cortex. Exp Neurol. 1990a;107:36–47. - PubMed
-
- Altman J, Bayer SA. Mosaic organization of the hippocampal neuroepithelium and the multiple germinal sources of dentate granule cells. J Comp Neurol. 1990b;301:325–42. - PubMed
-
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–35. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

