SIRT1 controls the transcription of the peroxisome proliferator-activated receptor-gamma Co-activator-1alpha (PGC-1alpha) gene in skeletal muscle through the PGC-1alpha autoregulatory loop and interaction with MyoD
- PMID: 19553684
- PMCID: PMC2755911
- DOI: 10.1074/jbc.M109.022749
SIRT1 controls the transcription of the peroxisome proliferator-activated receptor-gamma Co-activator-1alpha (PGC-1alpha) gene in skeletal muscle through the PGC-1alpha autoregulatory loop and interaction with MyoD
Abstract
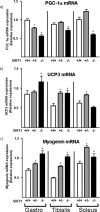
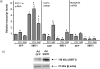
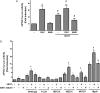
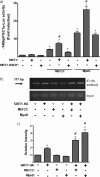
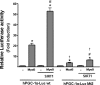
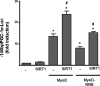
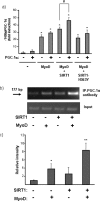
Peroxisome proliferator activated receptor-gamma co-activator-1alpha (PGC-1alpha) is a transcriptional co-activator that coordinately regulates the expression of distinct sets of metabolism-related genes in different tissues. Here we show that PGC-1alpha expression is reduced in skeletal muscles from mice lacking the sirtuin family deacetylase SIRT1. Conversely, SIRT1 activation or overexpression in differentiated C2C12 myotubes increased PGC-1alpha mRNA expression. The transcription-promoting effects of SIRT1 occurred through stimulation of PGC-1alpha promoter activity and were enhanced by co-transfection of myogenic factors, such as myocyte enhancer factor 2 (MEF2) and, especially, myogenic determining factor (MyoD). SIRT1 bound to the proximal promoter region of the PGC-1alpha gene, an interaction potentiated by MEF2C or MyoD, which also interact with this region. In the presence of MyoD, SIRT1 promoted a positive autoregulatory PGC-1alpha expression loop, such that overexpression of PGC-1alpha increased PGC-1alpha promoter activity in the presence of co-expressed MyoD and SIRT1. Chromatin immunoprecipitation showed that SIRT1 interacts with PGC-1alpha promoter and increases PGC-1alpha recruitment to its own promoter region. Immunoprecipitation assays further showed that SIRT1-PGC-1alpha interactions are enhanced by MyoD. Collectively, these data indicate that SIRT1 controls PGC-1alpha gene expression in skeletal muscle and that MyoD is a key mediator of this action. The involvement of MyoD in SIRT1-dependent PGC-1alpha expression may help to explain the ability of SIRT1 to drive muscle-specific gene expression and metabolism. Autoregulatory control of PGC-1alpha gene transcription seems to be a pivotal mechanism for conferring a transcription-activating response to SIRT1 in skeletal muscle.
Figures
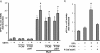

Similar articles
-
Real-time imaging of peroxisome proliferator-activated receptor-gamma coactivator-1alpha promoter activity in skeletal muscles of living mice.Am J Physiol Cell Physiol. 2004 Sep;287(3):C790-6. doi: 10.1152/ajpcell.00425.2003. Epub 2004 May 19. Am J Physiol Cell Physiol. 2004. PMID: 15151904
-
Myogenic basic helix-loop-helix proteins regulate the expression of peroxisomal proliferator activated receptor-gamma coactivator-1alpha.Endocrinology. 2006 Jun;147(6):3093-106. doi: 10.1210/en.2005-1317. Epub 2006 Mar 9. Endocrinology. 2006. PMID: 16527841
-
Sirtuin 1 (SIRT1) deacetylase activity is not required for mitochondrial biogenesis or peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1alpha) deacetylation following endurance exercise.J Biol Chem. 2011 Sep 2;286(35):30561-30570. doi: 10.1074/jbc.M111.261685. Epub 2011 Jul 11. J Biol Chem. 2011. PMID: 21757760 Free PMC article.
-
Deacetylation of PGC-1α by SIRT1: importance for skeletal muscle function and exercise-induced mitochondrial biogenesis.Appl Physiol Nutr Metab. 2011 Oct;36(5):589-97. doi: 10.1139/h11-070. Epub 2011 Sep 2. Appl Physiol Nutr Metab. 2011. PMID: 21888529 Review.
-
Exercise, PGC-1alpha, and metabolic adaptation in skeletal muscle.Appl Physiol Nutr Metab. 2009 Jun;34(3):424-7. doi: 10.1139/H09-030. Appl Physiol Nutr Metab. 2009. PMID: 19448709 Free PMC article. Review.
Cited by
-
Lactate Overload Inhibits Myogenic Activity in C2C12 Myotubes.Open Life Sci. 2019 Mar 20;14:29-37. doi: 10.1515/biol-2019-0004. eCollection 2019 Jan. Open Life Sci. 2019. PMID: 33817134 Free PMC article.
-
SIRT1 in forebrain excitatory neurons produces sexually dimorphic effects on depression-related behaviors and modulates neuronal excitability and synaptic transmission in the medial prefrontal cortex.Mol Psychiatry. 2020 May;25(5):1094-1111. doi: 10.1038/s41380-019-0352-1. Epub 2019 Jan 31. Mol Psychiatry. 2020. PMID: 30705425 Free PMC article.
-
Sirtuins in neurodegenerative diseases: an update on potential mechanisms.Front Aging Neurosci. 2013 Sep 25;5:53. doi: 10.3389/fnagi.2013.00053. Front Aging Neurosci. 2013. PMID: 24093018 Free PMC article. Review.
-
Erythropoietin alleviates hepatic insulin resistance via PPARγ-dependent AKT activation.Sci Rep. 2015 Dec 8;5:17878. doi: 10.1038/srep17878. Sci Rep. 2015. PMID: 26643367 Free PMC article.
-
Inverse regulation of the cytosolic Ca²⁺ buffer parvalbumin and mitochondrial volume in muscle cells via SIRT1/PGC-1α axis.PLoS One. 2012;7(9):e44837. doi: 10.1371/journal.pone.0044837. Epub 2012 Sep 13. PLoS One. 2012. PMID: 23028640 Free PMC article.
References
-
- Pilegaard H., Saltin B., Neufer P. D. (2003) Diabetes 52, 657–662 - PubMed
-
- Russell A. P., Feilchenfeldt J., Schreiber S., Praz M., Crettenand A., Gobelet C., Meier C. A., Bell D. R., Kralli A., Giacobino J. P., Dériaz O. (2003) Diabetes 52, 2874–2881 - PubMed
-
- Lin J., Wu H., Tarr P. T., Zhang C. Y., Wu Z., Boss O., Michael L. F., Puigserver P., Isotani E., Olson E. N., Lowell B. B., Bassel-Duby R., Spiegelman B. M. (2002) Nature 418, 797–801 - PubMed
-
- Chang J. H., Lin K. H., Shih C. H., Chang Y. J., Chi H. C., Chen S. L. (2006) Endocrinology 147, 3093–3106 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

