Innate immune sensing of modified vaccinia virus Ankara (MVA) is mediated by TLR2-TLR6, MDA-5 and the NALP3 inflammasome
- PMID: 19543380
- PMCID: PMC2691956
- DOI: 10.1371/journal.ppat.1000480
Innate immune sensing of modified vaccinia virus Ankara (MVA) is mediated by TLR2-TLR6, MDA-5 and the NALP3 inflammasome
Retraction in
-
Retraction: Innate Immune Sensing of Modified Vaccinia Virus Ankara (MVA) Is Mediated by TLR2-TLR6, MDA-5 and the NALP3 Inflammasome.PLoS Pathog. 2022 Jan 25;18(1):e1010263. doi: 10.1371/journal.ppat.1010263. eCollection 2022 Jan. PLoS Pathog. 2022. PMID: 35077525 Free PMC article. No abstract available.
Abstract
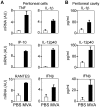
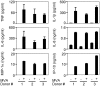
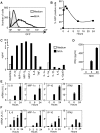
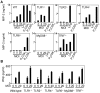
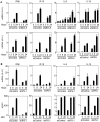
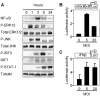
Modified vaccinia virus Ankara (MVA) is an attenuated double-stranded DNA poxvirus currently developed as a vaccine vector against HIV/AIDS. Profiling of the innate immune responses induced by MVA is essential for the design of vaccine vectors and for anticipating potential adverse interactions between naturally acquired and vaccine-induced immune responses. Here we report on innate immune sensing of MVA and cytokine responses in human THP-1 cells, primary human macrophages and mouse bone marrow-derived macrophages (BMDMs). The innate immune responses elicited by MVA in human macrophages were characterized by a robust chemokine production and a fairly weak pro-inflammatory cytokine response. Analyses of the cytokine production profile of macrophages isolated from knockout mice deficient in Toll-like receptors (TLRs) or in the adapter molecules MyD88 and TRIF revealed a critical role for TLR2, TLR6 and MyD88 in the production of IFNbeta-independent chemokines. MVA induced a marked up-regulation of the expression of RIG-I like receptors (RLR) and the IPS-1 adapter (also known as Cardif, MAVS or VISA). Reduced expression of RIG-I, MDA-5 and IPS-1 by shRNAs indicated that sensing of MVA by RLR and production of IFNbeta and IFNbeta-dependent chemokines was controlled by the MDA-5 and IPS-1 pathway in the macrophage. Crosstalk between TLR2-MyD88 and the NALP3 inflammasome was essential for expression and processing of IL-1beta. Transcription of the Il1b gene was markedly impaired in TLR2(-/-) and MyD88(-/-) BMDM, whereas mature and secreted IL-1beta was massively reduced in NALP3(-/-) BMDMs or in human THP-1 macrophages with reduced expression of NALP3, ASC or caspase-1 by shRNAs. Innate immune sensing of MVA and production of chemokines, IFNbeta and IL-1beta by macrophages is mediated by the TLR2-TLR6-MyD88, MDA-5-IPS-1 and NALP3 inflammasome pathways. Delineation of the host response induced by MVA is critical for improving our understanding of poxvirus antiviral escape mechanisms and for designing new MVA vaccine vectors with improved immunogenicity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

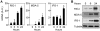

Similar articles
-
Improving Adaptive and Memory Immune Responses of an HIV/AIDS Vaccine Candidate MVA-B by Deletion of Vaccinia Virus Genes (C6L and K7R) Blocking Interferon Signaling Pathways.PLoS One. 2013 Jun 27;8(6):e66894. doi: 10.1371/journal.pone.0066894. Print 2013. PLoS One. 2013. PMID: 23826170 Free PMC article.
-
Deletion of the vaccinia virus N2L gene encoding an inhibitor of IRF3 improves the immunogenicity of modified vaccinia virus Ankara expressing HIV-1 antigens.J Virol. 2014 Mar;88(6):3392-410. doi: 10.1128/JVI.02723-13. Epub 2014 Jan 3. J Virol. 2014. PMID: 24390336 Free PMC article.
-
Cytosolic double-stranded RNA activates the NLRP3 inflammasome via MAVS-induced membrane permeabilization and K+ efflux.J Immunol. 2014 Oct 15;193(8):4214-4222. doi: 10.4049/jimmunol.1400582. Epub 2014 Sep 15. J Immunol. 2014. PMID: 25225670 Free PMC article.
-
Modified Vaccinia virus Ankara: innate immune activation and induction of cellular signalling.Vaccine. 2013 Sep 6;31(39):4231-4. doi: 10.1016/j.vaccine.2013.03.017. Epub 2013 Mar 21. Vaccine. 2013. PMID: 23523404 Review.
-
Mechanisms and pathways of innate immune activation and regulation in health and cancer.Hum Vaccin Immunother. 2014;10(11):3270-85. doi: 10.4161/21645515.2014.979640. Hum Vaccin Immunother. 2014. PMID: 25625930 Free PMC article. Review.
Cited by
-
International Union of Basic and Clinical Pharmacology. XCVI. Pattern recognition receptors in health and disease.Pharmacol Rev. 2015;67(2):462-504. doi: 10.1124/pr.114.009928. Pharmacol Rev. 2015. PMID: 25829385 Free PMC article. Review.
-
Roles for Treg expansion and HMGB1 signaling through the TLR1-2-6 axis in determining the magnitude of the antigen-specific immune response to MVA85A.PLoS One. 2013 Jul 3;8(7):e67922. doi: 10.1371/journal.pone.0067922. Print 2013. PLoS One. 2013. PMID: 23844129 Free PMC article.
-
The role of inflammasome modulation in virulence.Virulence. 2012 May 1;3(3):262-70. doi: 10.4161/viru.20266. Epub 2012 May 1. Virulence. 2012. PMID: 22546900 Free PMC article. Review.
-
Cytosolic surveillance and antiviral immunity.Curr Opin Virol. 2011 Dec;1(6):455-62. doi: 10.1016/j.coviro.2011.11.004. Epub 2011 Dec 4. Curr Opin Virol. 2011. PMID: 22440909 Free PMC article. Review.
-
Central roles of NLRs and inflammasomes in viral infection.Nat Rev Immunol. 2010 Oct;10(10):688-98. doi: 10.1038/nri2851. Epub 2010 Sep 17. Nat Rev Immunol. 2010. PMID: 20847744 Free PMC article. Review.
References
-
- Gomez CE, Najera JL, Krupa M, Esteban M. The poxvirus vectors MVA and NYVAC as gene delivery systems for vaccination against infectious diseases and cancer. Curr Gene Ther. 2008;8:97–120. - PubMed
-
- Artenstein AW. New generation smallpox vaccines: a review of preclinical and clinical data. Rev Med Virol. 2008;18:217–231. - PubMed
-
- Johnston MI, Fauci AS. An HIV vaccine–evolving concepts. N Engl J Med. 2007;356:2073–2081. - PubMed
-
- Johnston MI, Fauci AS. An HIV vaccine–challenges and prospects. N Engl J Med. 2008;359:888–890. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

