The role of thiols and disulfides on protein stability
- PMID: 19538140
- PMCID: PMC3319691
- DOI: 10.2174/138920309789630534
The role of thiols and disulfides on protein stability
Abstract
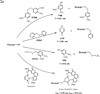
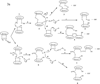
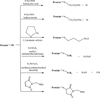
There has been a tremendous increase in the number of approved drugs derived from recombinant proteins; however, their development as potential drugs has been hampered by their instability that causes difficulty to formulate them as therapeutic agents. It has been shown that the reactivity of thiol and disulfide functional groups could catalyze chemical (i.e., oxidation and beta-elimination reactions) and physical (i.e., aggregation and precipitation) degradations of proteins. Because most proteins contain a free Cys residue or/and a disulfide bond, this review is focused on their roles in the physical and chemical stability of proteins. The effect of introducing a disulfide bond to improve physical stability of proteins and the mechanisms of degradation of disulfide bond were discussed. The qualitative/quantitative methods to determine the presence of thiol in the Cys residue and various methods to derivatize thiol group for improving protein stability were also illustrated.
Figures




Similar articles
-
Thiol-Disulfide Exchange in Human Growth Hormone.Pharm Res. 2016 Jun;33(6):1370-82. doi: 10.1007/s11095-016-1879-3. Epub 2016 Feb 17. Pharm Res. 2016. PMID: 26887678 Free PMC article.
-
How the disulfide conformation determines the disulfide/thiol redox potential.J Biomol Struct Dyn. 2015;33(1):93-103. doi: 10.1080/07391102.2013.851034. Epub 2013 Nov 21. J Biomol Struct Dyn. 2015. PMID: 24256142
-
Effects of aromatic thiols on thiol-disulfide interchange reactions that occur during protein folding.J Org Chem. 2001 Jun 15;66(12):4244-9. doi: 10.1021/jo015600a. J Org Chem. 2001. PMID: 11397160
-
Quantification of thiols and disulfides.Biochim Biophys Acta. 2014 Feb;1840(2):838-46. doi: 10.1016/j.bbagen.2013.03.031. Epub 2013 Apr 6. Biochim Biophys Acta. 2014. PMID: 23567800 Free PMC article. Review.
-
Thiol-Disulfide Exchange Reactions in the Mammalian Extracellular Environment.Annu Rev Chem Biomol Eng. 2016 Jun 7;7:197-222. doi: 10.1146/annurev-chembioeng-080615-033553. Epub 2016 Mar 17. Annu Rev Chem Biomol Eng. 2016. PMID: 27023663 Free PMC article. Review.
Cited by
-
Diverse roles of low-molecular weight thiol GSH in Francisella's virulence, location sensing and GSH-stealing from host.Curr Res Microb Sci. 2023 Dec 20;6:100218. doi: 10.1016/j.crmicr.2023.100218. eCollection 2024. Curr Res Microb Sci. 2023. PMID: 38303966 Free PMC article.
-
Antibody Aggregation: Insights from Sequence and Structure.Antibodies (Basel). 2016 Sep 5;5(3):19. doi: 10.3390/antib5030019. Antibodies (Basel). 2016. PMID: 31558000 Free PMC article. Review.
-
Effects of PCSK9 Targeting: Alleviating Oxidation, Inflammation, and Atherosclerosis.J Am Heart Assoc. 2022 Feb;11(3):e023328. doi: 10.1161/JAHA.121.023328. Epub 2022 Jan 20. J Am Heart Assoc. 2022. PMID: 35048716 Free PMC article. Review.
-
Engineered control of enzyme structural dynamics and function.Protein Sci. 2018 Apr;27(4):825-838. doi: 10.1002/pro.3379. Epub 2018 Feb 16. Protein Sci. 2018. PMID: 29380452 Free PMC article. Review.
-
Nitrosylation vs. oxidation - How to modulate cold physical plasmas for biological applications.PLoS One. 2019 May 8;14(5):e0216606. doi: 10.1371/journal.pone.0216606. eCollection 2019. PLoS One. 2019. PMID: 31067274 Free PMC article.
References
-
- Guide to Biotechnology, editor. Bio. Approved Biotechnology Drugs. 2007.
-
- Cyran R. The market for bioengineered protein drugs BCC Research Report and Reviews. ed. 2004.
-
- Nonoyama A, Laurence JS, Garriques L, Qi H, Le T, Middaugh CR. A biophysical characterization of the peptide drug pramlintide (AC137) using empirical phase diagrams. Journal of Pharmaceutical Sciences. 2007 - PubMed
-
- Fan H, Li H, Zhang M, Middaugh CR. Effects of solutes on empirical phase diagrams of human fibroblast growth factor 1. J Pharm Sci. 2007;96:1490–1503. - PubMed
-
- Brandau DT, Joshi SB, Smalter AM, Kim S, Steadman B, Middaugh CR. Stability of the Clostridium botulinum type A neurotoxin complex: an empirical phase diagram based approach. Mol Pharm. 2007;4:571–582. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
