HMGB1 is markedly elevated within 6 hours of mechanical trauma in humans
- PMID: 19533845
- PMCID: PMC4097145
- DOI: 10.1097/shk.0b013e3181997173
HMGB1 is markedly elevated within 6 hours of mechanical trauma in humans
Abstract
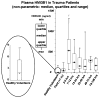
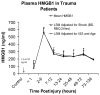
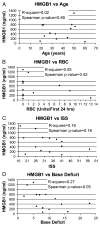
High-mobility group box 1 (HMGB1) is a late mediator of the systemic inflammation associated with sepsis. Recently, HMGB1 has been shown in animals to be a mediator of hemorrhage-induced organ dysfunction. However, the time course of plasma HMGB1 elevations after trauma in humans remains to be elucidated. Consequently, we hypothesized that mechanical trauma in humans would result in early significant elevations of plasma HMGB1. Trauma patients at risk for multiple organ failure (ISS > or = 15) were identified for inclusion (n = 23), and postinjury plasma samples were assayed for HMGB1 by enzyme-linked immunosorbent assay. Comparison of postinjury HMGB1 levels with markers for patient outcome (age, injury severity score, units of red blood cell (RBC) transfused per first 24 h, and base deficit) was performed. To investigate whether postinjury transfusion contributes to elevations of circulating HMGB1, levels were determined in both leuko-reduced and non-leuko-reduced packed RBCs. Plasma HMGB1 was elevated more than 30-fold above healthy controls within 1 h of injury (median, 57.76 vs. 1.77 ng/mL; P < 0.003), peaked from 2 to 6 h postinjury (median, 526.18 ng/mL; P < 0.01 vs. control), and remained elevated above control through 136 h. No clear relationship was evident between postinjury HMGB1 levels and markers for patient outcome. High-mobility group box 1 levels increase with duration of RBC storage, although concentrations did not account for postinjury plasma levels. Leuko-reduced attenuated HMGB1 levels in packed RBCs by approximately 55% (P < 0.01). Plasma HMGB1 is significantly increased within 1 h of trauma in humans with marked elevations occurring from 2 to 6 h postinjury. These results suggest that, in contrast to sepsis, HMGB1 release is an early event after traumatic injury in humans. Thus, HMGB1 may be integral to the early inflammatory response to trauma and is a potential target for future therapeutics.
Figures

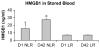
Similar articles
-
High plasma levels of high mobility group box 1 is associated with the risk of sepsis in severe blunt chest trauma patients: a prospective cohort study.J Cardiothorac Surg. 2014 Aug 2;9:133. doi: 10.1186/s13019-014-0133-5. J Cardiothorac Surg. 2014. PMID: 25085006 Free PMC article.
-
Role of heme in lung bacterial infection after trauma hemorrhage and stored red blood cell transfusion: A preclinical experimental study.PLoS Med. 2018 Mar 9;15(3):e1002522. doi: 10.1371/journal.pmed.1002522. eCollection 2018 Mar. PLoS Med. 2018. PMID: 29522519 Free PMC article.
-
Early release of high mobility group box nuclear protein 1 after severe trauma in humans: role of injury severity and tissue hypoperfusion.Crit Care. 2009;13(6):R174. doi: 10.1186/cc8152. Epub 2009 Nov 4. Crit Care. 2009. PMID: 19887013 Free PMC article.
-
Red blood cell storage duration and trauma.Transfus Med Rev. 2015 Apr;29(2):120-6. doi: 10.1016/j.tmrv.2014.09.007. Epub 2014 Dec 18. Transfus Med Rev. 2015. PMID: 25573415 Review.
-
High-dimensional proteomics identifies organ injury patterns associated with outcomes in human trauma.J Trauma Acute Care Surg. 2023 Jun 1;94(6):803-813. doi: 10.1097/TA.0000000000003880. Epub 2023 Feb 13. J Trauma Acute Care Surg. 2023. PMID: 36787435 Free PMC article. Review.
Cited by
-
An ongoing search for potential targets and therapies for lethal sepsis.Mil Med Res. 2015 Aug 8;2:20. doi: 10.1186/s40779-015-0047-0. eCollection 2015. Mil Med Res. 2015. PMID: 26257917 Free PMC article.
-
Evidence for SIRT1 Mediated HMGB1 Release From Kidney Cells in the Early Stages of Hemorrhagic Shock.Front Physiol. 2019 Jul 5;10:854. doi: 10.3389/fphys.2019.00854. eCollection 2019. Front Physiol. 2019. PMID: 31333497 Free PMC article.
-
The RAGE axis in systemic inflammation, acute lung injury and myocardial dysfunction: an important therapeutic target?Intensive Care Med. 2010 Oct;36(10):1644-1656. doi: 10.1007/s00134-010-1952-z. Epub 2010 Jul 15. Intensive Care Med. 2010. PMID: 20631986 Review.
-
Targeting Oxidative Stress with Antioxidant Duotherapy after Experimental Traumatic Brain Injury.Int J Mol Sci. 2021 Sep 29;22(19):10555. doi: 10.3390/ijms221910555. Int J Mol Sci. 2021. PMID: 34638900 Free PMC article.
-
Alarmin HMGB1 is released in the small intestine of gnotobiotic piglets infected with enteric pathogens and its level in plasma reflects severity of sepsis.J Clin Immunol. 2011 Jun;31(3):488-97. doi: 10.1007/s10875-010-9505-3. Epub 2011 Jan 12. J Clin Immunol. 2011. PMID: 21225449
References
-
- Goodwin GH, Sanders C, Johns EW. A new group of chromatin-associated proteins with a high content of acidic and basic amino acids. Eur J Biochem. 1973;38(1):14–19. - PubMed
-
- Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285(5425):248–251. - PubMed
-
- Rouhiainen A, Tumova S, Valmu L, Kalkkinen N, Rauvala H. Pivotal advance: analysis of proinflammatory activity of highly purified eukaryotic recombinant HMGB1 (amphoterin) J Leukoc Biol. 2007;81(1):49–58. - PubMed
-
- Sha Y, Zmijewski J, Xu Z, Abraham E. HMGB1 develops enhanced proinflammatory activity by binding to cytokines. J Immunol. 2008;180(4):2531–2537. - PubMed
-
- Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, et al. Toll-like receptor 9–dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8(5):487–496. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

