The effects of nitroxyl (HNO) on soluble guanylate cyclase activity: interactions at ferrous heme and cysteine thiols
- PMID: 19531488
- PMCID: PMC2755905
- DOI: 10.1074/jbc.M109.014282
The effects of nitroxyl (HNO) on soluble guanylate cyclase activity: interactions at ferrous heme and cysteine thiols
Abstract
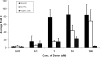
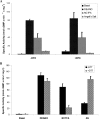
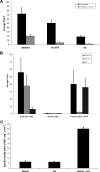
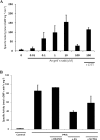
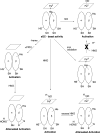
It has been previously proposed that nitric oxide (NO) is the only biologically relevant nitrogen oxide capable of activating the enzyme soluble guanylate cyclase (sGC). However, recent reports implicate HNO as another possible activator of sGC. Herein, we examine the affect of HNO donors on the activity of purified bovine lung sGC and find that, indeed, HNO is capable of activating this enzyme. Like NO, HNO activation appears to occur via interaction with the regulatory ferrous heme on sGC. Somewhat unexpectedly, HNO does not activate the ferric form of the enzyme. Finally, HNO-mediated cysteine thiol modification appears to also affect enzyme activity leading to inhibition. Thus, sGC activity can be regulated by HNO via interactions at both the regulatory heme and cysteine thiols.
Figures
Similar articles
-
The concomitant coronary vasodilator and positive inotropic actions of the nitroxyl donor Angeli's salt in the intact rat heart: contribution of soluble guanylyl cyclase-dependent and -independent mechanisms.Br J Pharmacol. 2014 Apr;171(7):1722-34. doi: 10.1111/bph.12568. Br J Pharmacol. 2014. PMID: 24372173 Free PMC article.
-
Soluble guanylate cyclase is required for systemic vasodilation but not positive inotropy induced by nitroxyl in the mouse.Hypertension. 2015 Feb;65(2):385-92. doi: 10.1161/HYPERTENSIONAHA.114.04285. Epub 2014 Dec 1. Hypertension. 2015. PMID: 25452469 Free PMC article.
-
Mechanisms underlying activation of soluble guanylate cyclase by the nitroxyl donor Angeli's salt.Mol Pharmacol. 2009 Nov;76(5):1115-22. doi: 10.1124/mol.109.059915. Epub 2009 Aug 31. Mol Pharmacol. 2009. PMID: 19720727
-
Thiol-Based Redox Modulation of Soluble Guanylyl Cyclase, the Nitric Oxide Receptor.Antioxid Redox Signal. 2017 Jan 20;26(3):137-149. doi: 10.1089/ars.2015.6591. Epub 2016 Apr 1. Antioxid Redox Signal. 2017. PMID: 26906466 Free PMC article. Review.
-
Nitric oxide-independent stimulation of soluble guanylate cyclase with BAY 41-2272 in cardiovascular disease.Cardiovasc Drug Rev. 2007 Spring;25(1):30-45. doi: 10.1111/j.1527-3466.2007.00003.x. Cardiovasc Drug Rev. 2007. PMID: 17445086 Review.
Cited by
-
Computational investigations of HNO in biology.J Inorg Biochem. 2013 Jan;118:191-200. doi: 10.1016/j.jinorgbio.2012.09.023. Epub 2012 Oct 5. J Inorg Biochem. 2013. PMID: 23103077 Free PMC article. Review.
-
Acyloxy nitroso compounds as nitroxyl (HNO) donors: kinetics, reactions with thiols, and vasodilation properties.J Med Chem. 2011 Feb 24;54(4):1059-70. doi: 10.1021/jm101432z. Epub 2011 Jan 19. J Med Chem. 2011. PMID: 21247168 Free PMC article.
-
Nitroxyl: A Novel Strategy to Circumvent Diabetes Associated Impairments in Nitric Oxide Signaling.Front Pharmacol. 2020 May 19;11:727. doi: 10.3389/fphar.2020.00727. eCollection 2020. Front Pharmacol. 2020. PMID: 32508651 Free PMC article. Review.
-
Basic principles and emerging concepts in the redox control of transcription factors.Antioxid Redox Signal. 2011 Oct 15;15(8):2335-81. doi: 10.1089/ars.2010.3534. Epub 2011 Apr 5. Antioxid Redox Signal. 2011. PMID: 21194351 Free PMC article. Review.
-
Cardiovascular consequences when nitric oxide and lipid signaling converge.Circ Res. 2009 Sep 11;105(6):511-22. doi: 10.1161/CIRCRESAHA.109.202077. Circ Res. 2009. PMID: 19745170 Free PMC article. Review.
References
-
- Hobbs A. J., Ignarro L. J. (1996) Methods Enzymol. 269, 134–148 - PubMed
-
- Denninger J. W., Marletta M. A. (1999) Biochim. Biophys. Acta 1411, 334–350 - PubMed
-
- Ignarro L. J., Degnan J. N., Baricos W. H., Kadowitz P. J., Wolin M. S. (1982) Biochim. Biophys. Acta 718, 49–59 - PubMed
-
- Burstyn J. N., Yu A. E., Dierks E. A., Hawkins B. K., Dawson J. H. (1995) Biochemistry 34, 5896–5903 - PubMed
-
- DeRubertis F. R., Craven P. A. (1977) J. Biol. Chem. 252, 5804–5814 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

