CCR3 is a target for age-related macular degeneration diagnosis and therapy
- PMID: 19525930
- PMCID: PMC2712122
- DOI: 10.1038/nature08151
CCR3 is a target for age-related macular degeneration diagnosis and therapy
Abstract
Age-related macular degeneration (AMD), a leading cause of blindness worldwide, is as prevalent as cancer in industrialized nations. Most blindness in AMD results from invasion of the retina by choroidal neovascularisation (CNV). Here we show that the eosinophil/mast cell chemokine receptor CCR3 is specifically expressed in choroidal neovascular endothelial cells in humans with AMD, and that despite the expression of its ligands eotaxin-1, -2 and -3, neither eosinophils nor mast cells are present in human CNV. Genetic or pharmacological targeting of CCR3 or eotaxins inhibited injury-induced CNV in mice. CNV suppression by CCR3 blockade was due to direct inhibition of endothelial cell proliferation, and was uncoupled from inflammation because it occurred in mice lacking eosinophils or mast cells, and was independent of macrophage and neutrophil recruitment. CCR3 blockade was more effective at reducing CNV than vascular endothelial growth factor A (VEGF-A) neutralization, which is in clinical use at present, and, unlike VEGF-A blockade, is not toxic to the mouse retina. In vivo imaging with CCR3-targeting quantum dots located spontaneous CNV invisible to standard fluorescein angiography in mice before retinal invasion. CCR3 targeting might reduce vision loss due to AMD through early detection and therapeutic angioinhibition.
Conflict of interest statement
Figures
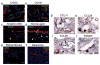
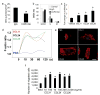

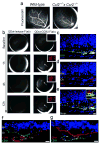
Comment in
-
Vision: New light on allergy receptor.Nature. 2009 Jul 9;460(7252):182-3. doi: 10.1038/460182a. Nature. 2009. PMID: 19587753 No abstract available.
Similar articles
-
Vision: New light on allergy receptor.Nature. 2009 Jul 9;460(7252):182-3. doi: 10.1038/460182a. Nature. 2009. PMID: 19587753 No abstract available.
-
Retinal Inhibition of CCR3 Induces Retinal Cell Death in a Murine Model of Choroidal Neovascularization.PLoS One. 2016 Jun 16;11(6):e0157748. doi: 10.1371/journal.pone.0157748. eCollection 2016. PLoS One. 2016. PMID: 27309355 Free PMC article.
-
Suppression of laser-induced choroidal neovascularization by a CCR3 antagonist.Invest Ophthalmol Vis Sci. 2013 Feb 28;54(2):1564-72. doi: 10.1167/iovs.11-9095. Invest Ophthalmol Vis Sci. 2013. PMID: 23404125
-
Eotaxins and CCR3 receptor in inflammatory and allergic skin diseases: therapeutical implications.Curr Drug Targets Inflamm Allergy. 2003 Mar;2(1):81-94. doi: 10.2174/1568010033344480. Curr Drug Targets Inflamm Allergy. 2003. PMID: 14561178 Review.
-
An emerging role for eotaxins in neurodegenerative disease.Clin Immunol. 2018 Apr;189:29-33. doi: 10.1016/j.clim.2016.09.010. Epub 2016 Sep 21. Clin Immunol. 2018. PMID: 27664933 Review.
Cited by
-
Study in vivo intraocular biocompatibility of in situ gelation hydrogels: poly(2-ethyl oxazoline)-block-poly(ε-caprolactone)-block-poly(2-ethyl oxazoline) copolymer, matrigel and pluronic F127.PLoS One. 2013 Jul 1;8(7):e67495. doi: 10.1371/journal.pone.0067495. Print 2013. PLoS One. 2013. PMID: 23840873 Free PMC article.
-
Bioinformatical Analysis of miRNA-mRNA Interaction Network Underlying Macrophage Aging and Cholesterol-Responsive Difference between Young and Aged Macrophages.Biomed Res Int. 2020 Jun 12;2020:9267475. doi: 10.1155/2020/9267475. eCollection 2020. Biomed Res Int. 2020. PMID: 32626771 Free PMC article.
-
Protein Microarrays for Ocular Diseases.Methods Mol Biol. 2021;2344:239-265. doi: 10.1007/978-1-0716-1562-1_17. Methods Mol Biol. 2021. PMID: 34115364 Review.
-
Profiling disease-selective drug targets: From proteomics to ligandomics.Drug Discov Today. 2023 Mar;28(3):103430. doi: 10.1016/j.drudis.2022.103430. Epub 2022 Nov 4. Drug Discov Today. 2023. PMID: 36343915 Free PMC article. Review.
-
Anti-angiogenic Therapy for Retinal Disease.Handb Exp Pharmacol. 2017;242:271-307. doi: 10.1007/164_2016_78. Handb Exp Pharmacol. 2017. PMID: 27783271 Free PMC article.
References
-
- Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP. Age–related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol. 2003;48:257–293. - PubMed
-
- Gragoudas ES, Adamis AP, Cunningham ET, Jr, Feinsod M, Guyer DR. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351:2805–2816. - PubMed
-
- Brown DM, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–1444. - PubMed
-
- Rosenfeld PJ, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. - PubMed
-
- Famiglietti EV, et al. Immunocytochemical localization of vascular endothelial growth factor in neurons and glial cells of human retina. Brain Res. 2003;969:195–204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 EY015422/EY/NEI NIH HHS/United States
- R37 AI045898/AI/NIAID NIH HHS/United States
- R01 EY015422-04/EY/NEI NIH HHS/United States
- R01 EY018836-02/EY/NEI NIH HHS/United States
- R01 DK076893/DK/NIDDK NIH HHS/United States
- EY010572/EY/NEI NIH HHS/United States
- EY017182/EY/NEI NIH HHS/United States
- EY015422/EY/NEI NIH HHS/United States
- DK076893/DK/NIDDK NIH HHS/United States
- R01 EY018350/EY/NEI NIH HHS/United States
- EY018836/EY/NEI NIH HHS/United States
- EY015130/EY/NEI NIH HHS/United States
- R01 EY018836/EY/NEI NIH HHS/United States
- R01 EY018350-02/EY/NEI NIH HHS/United States
- AI039759/AI/NIAID NIH HHS/United States
- AI45898/AI/NIAID NIH HHS/United States
- EY017011/EY/NEI NIH HHS/United States
- EY017950/EY/NEI NIH HHS/United States
- EY018350/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

