Interaction of reelin with amyloid precursor protein promotes neurite outgrowth
- PMID: 19515914
- PMCID: PMC2759694
- DOI: 10.1523/JNEUROSCI.4872-08.2009
Interaction of reelin with amyloid precursor protein promotes neurite outgrowth
Abstract
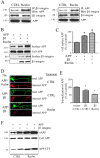
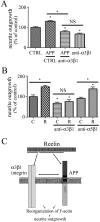
The processing of amyloid precursor protein (APP) to Abeta is an important event in the pathogenesis of Alzheimer's disease, but the physiological function of APP is not well understood. Our previous work has shown that APP processing and Abeta production are regulated by the extracellular matrix protein Reelin. In the present study, we examined whether Reelin interacts with APP, and the functional consequences of that interaction in vitro. Using coimmunoprecipitation, we found that Reelin interacted with APP through the central domain of Reelin (repeats 3-6) and the E1 extracellular domain of APP. Reelin increased cell surface levels of APP and decreased endocytosis of APP in hippocampal neurons in vitro. In vivo, Reelin levels were increased in brains of APP knock-out mice and decreased in APP-overexpressing mice. RNA interference knockdown of APP decreased neurite outgrowth in vitro and prevented Reelin from increasing neurite outgrowth. Knock-out of APP or Reelin decreased dendritic arborization in cortical neurons in vivo, and APP overexpression increased dendritic arborization. APP and Reelin have previously been shown to promote neurite outgrowth through interactions with integrins. We confirmed that APP interacted with alpha3beta1 integrin, and alpha3beta1 integrin altered APP trafficking and processing. Addition of an alpha3beta1 integrin antibody prevented APP and Reelin-induced neurite outgrowth. These findings demonstrate that Reelin interacts with APP, potentially having important effects on neurite development.
Figures


Similar articles
-
DAB1 and Reelin effects on amyloid precursor protein and ApoE receptor 2 trafficking and processing.J Biol Chem. 2006 Nov 17;281(46):35176-85. doi: 10.1074/jbc.M602162200. Epub 2006 Sep 1. J Biol Chem. 2006. PMID: 16951405
-
Reduced Reelin expression accelerates amyloid-beta plaque formation and tau pathology in transgenic Alzheimer's disease mice.J Neurosci. 2010 Jul 7;30(27):9228-40. doi: 10.1523/JNEUROSCI.0418-10.2010. J Neurosci. 2010. PMID: 20610758 Free PMC article.
-
Amyloid precursor protein cytoplasmic domain antagonizes reelin neurite outgrowth inhibition of hippocampal neurons.Neurobiol Aging. 2008 Apr;29(4):542-53. doi: 10.1016/j.neurobiolaging.2006.11.012. Epub 2006 Dec 13. Neurobiol Aging. 2008. PMID: 17169463
-
The involvement of Reelin in neurodevelopmental disorders.Neuropharmacology. 2013 May;68:122-35. doi: 10.1016/j.neuropharm.2012.08.015. Epub 2012 Sep 7. Neuropharmacology. 2013. PMID: 22981949 Free PMC article. Review.
-
Reelin links Apolipoprotein E4, Tau, and Amyloid-β in Alzheimer's disease.Ageing Res Rev. 2024 Jul;98:102339. doi: 10.1016/j.arr.2024.102339. Epub 2024 May 14. Ageing Res Rev. 2024. PMID: 38754634 Review.
Cited by
-
Guided exploration of genomic risk for gray matter abnormalities in schizophrenia using parallel independent component analysis with reference.Neuroimage. 2013 Dec;83:384-96. doi: 10.1016/j.neuroimage.2013.05.073. Epub 2013 May 28. Neuroimage. 2013. PMID: 23727316 Free PMC article.
-
Pancortins interact with amyloid precursor protein and modulate cortical cell migration.Development. 2012 Nov;139(21):3986-96. doi: 10.1242/dev.082909. Epub 2012 Sep 19. Development. 2012. PMID: 22992957 Free PMC article.
-
Soluble amyloid precursor protein (APP) regulates transthyretin and Klotho gene expression without rescuing the essential function of APP.Proc Natl Acad Sci U S A. 2010 Oct 5;107(40):17362-7. doi: 10.1073/pnas.1012568107. Epub 2010 Sep 20. Proc Natl Acad Sci U S A. 2010. PMID: 20855613 Free PMC article.
-
Neurogenesis and Alzheimer's disease: at the crossroads.Exp Neurol. 2010 Jun;223(2):267-81. doi: 10.1016/j.expneurol.2009.08.009. Epub 2009 Aug 19. Exp Neurol. 2010. PMID: 19699201 Free PMC article. Review.
-
Follicular fluid Aβ40 concentrations may be associated with ongoing pregnancy following in vitro fertilization.J Assist Reprod Genet. 2014 Dec;31(12):1611-20. doi: 10.1007/s10815-014-0345-6. Epub 2014 Sep 21. J Assist Reprod Genet. 2014. PMID: 25241131 Free PMC article.
References
-
- Andressen C, Arnhold S, Puschmann M, Bloch W, Hescheler J, Fässler R, Addicks K. Beta1 integrin deficiency impairs migration and differentiation of mouse embryonic stem cell derived neurons. Neurosci Lett. 1998;251:165–168. - PubMed
-
- Beffert U, Weeber EJ, Durudas A, Qiu S, Masiulis I, Sweatt JD, Li WP, Adelmann G, Frotscher M, Hammer RE, Herz J. Modulation of synaptic plasticity and memory by Reelin involves differential splicing of the lipoprotein receptor Apoer2. Neuron. 2005;47:567–579. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG014473-13/AG/NIA NIH HHS/United States
- R03 AG030060-02/AG/NIA NIH HHS/United States
- AG032330/AG/NIA NIH HHS/United States
- R01 AG035355/AG/NIA NIH HHS/United States
- R01 NS048085-04/NS/NINDS NIH HHS/United States
- R29 AG014473/AG/NIA NIH HHS/United States
- R37 AG012406/AG/NIA NIH HHS/United States
- R01 NS048085/NS/NINDS NIH HHS/United States
- R01 AG027924/AG/NIA NIH HHS/United States
- P01 AG030128/AG/NIA NIH HHS/United States
- AG014473/AG/NIA NIH HHS/United States
- R01 AG014473/AG/NIA NIH HHS/United States
- R03 AG030060/AG/NIA NIH HHS/United States
- R03 AG032330/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
