Simian virus 40 small T antigen activates AMPK and triggers autophagy to protect cancer cells from nutrient deprivation
- PMID: 19515765
- PMCID: PMC2738183
- DOI: 10.1128/JVI.00603-09
Simian virus 40 small T antigen activates AMPK and triggers autophagy to protect cancer cells from nutrient deprivation
Abstract
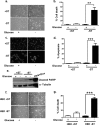
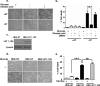
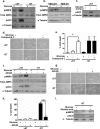
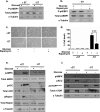
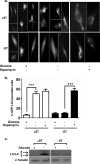
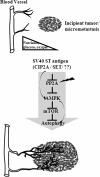
As tumors grow larger, they often experience an insufficient supply of oxygen and nutrients. Hence, cancer cells must develop mechanisms to overcome these stresses. Using an in vitro transformation model where the presence of the simian virus 40 (SV40) small T (ST) antigen has been shown to be critical for tumorigenic transformation, we investigated whether the ST antigen has a role to play in regulating the energy homeostasis of cancer cells. We find that cells expressing the SV40 ST antigen (+ST cells) are more resistant to glucose deprivation-induced cell death than cells lacking the SV40 ST antigen (-ST cells). Mechanistically, we find that the ST antigen mediates this effect by activating a nutrient-sensing kinase, AMP-activated protein kinase (AMPK). The basal level of active, phosphorylated AMPK was higher in +ST cells than in -ST cells, and these levels increased further in response to glucose deprivation. Additionally, inhibition of AMPK in +ST cells increased the rate of cell death, while activation of AMPK in -ST cells decreased the rate of cell death, under conditions of glucose deprivation. We further show that AMPK mediates its effects, at least in part, by inhibiting mTOR (mammalian target of rapamycin), thereby shutting down protein translation. Finally, we show that +ST cells exhibit a higher percentage of autophagy than -ST cells upon glucose deprivation. Thus, we demonstrate a novel role for the SV40 ST antigen in cancers, where it functions to maintain energy homeostasis during glucose deprivation by activating AMPK, inhibiting mTOR, and inducing autophagy as an alternate energy source.
Figures
Similar articles
-
Schizandrin Protects against OGD/R-Induced Neuronal Injury by Suppressing Autophagy: Involvement of the AMPK/mTOR Pathway.Molecules. 2019 Oct 8;24(19):3624. doi: 10.3390/molecules24193624. Molecules. 2019. PMID: 31597329 Free PMC article.
-
Impaired Cellular Energy Metabolism Contributes to Duck-Enteritis-Virus-Induced Autophagy via the AMPK-TSC2-MTOR Signaling Pathway.Front Cell Infect Microbiol. 2017 Sep 26;7:423. doi: 10.3389/fcimb.2017.00423. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 29018776 Free PMC article.
-
Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy.Circ Res. 2007 Mar 30;100(6):914-22. doi: 10.1161/01.RES.0000261924.76669.36. Epub 2007 Mar 1. Circ Res. 2007. PMID: 17332429
-
Role of AMP-activated protein kinase in cancer therapy.Arch Pharm (Weinheim). 2014 Jul;347(7):457-68. doi: 10.1002/ardp.201300402. Epub 2014 Mar 28. Arch Pharm (Weinheim). 2014. PMID: 24677093 Review.
-
LKB1 and AMP-activated protein kinase control of mTOR signalling and growth.Acta Physiol (Oxf). 2009 May;196(1):65-80. doi: 10.1111/j.1748-1716.2009.01972.x. Epub 2009 Feb 19. Acta Physiol (Oxf). 2009. PMID: 19245654 Free PMC article. Review.
Cited by
-
Transformation with oncogenic Ras and the simian virus 40 T antigens induces caspase-dependent sensitivity to fatty acid biosynthetic inhibition.J Virol. 2015 Jun;89(12):6406-17. doi: 10.1128/JVI.03671-14. Epub 2015 Apr 8. J Virol. 2015. PMID: 25855740 Free PMC article.
-
AMP-activated Protein Kinase As a Target For Pathogens: Friends Or Foes?Curr Drug Targets. 2016;17(8):942-53. doi: 10.2174/1389450116666150416120559. Curr Drug Targets. 2016. PMID: 25882224 Free PMC article. Review.
-
Significance of Autophagy in Dengue Virus Infection: A Brief Review.Am J Trop Med Hyg. 2019 Apr;100(4):783-790. doi: 10.4269/ajtmh.18-0761. Am J Trop Med Hyg. 2019. PMID: 30761986 Free PMC article. Review.
-
Orchestrated efforts on host network hijacking: Processes governing virus replication.Virulence. 2020 Dec;11(1):183-198. doi: 10.1080/21505594.2020.1726594. Virulence. 2020. PMID: 32050846 Free PMC article. Review.
-
Serum depletion induces changes in protein expression in the trophoblast-derived cell line HTR-8/SVneo.Cell Mol Biol Lett. 2016 Oct 16;21:22. doi: 10.1186/s11658-016-0018-9. eCollection 2016. Cell Mol Biol Lett. 2016. PMID: 28536624 Free PMC article.
References
-
- Ali, S. H., and J. A. DeCaprio. 2001. Cellular transformation by SV40 large T antigen: interaction with host proteins. Semin. Cancer Biol. 1115-23. - PubMed
-
- Arroyo, J. D., and W. C. Hahn. 2005. Involvement of PP2A in viral and cellular transformation. Oncogene 247746-7755. - PubMed
-
- Carling, D. 2005. AMP-activated protein kinase: balancing the scales. Biochimie 8787-91. - PubMed
-
- Chellappan, S., V. B. Kraus, B. Kroger, K. Munger, P. M. Howley, W. C. Phelps, and J. R. Nevins. 1992. Adenovirus E1A, simian virus 40 tumor antigen, and human papillomavirus E7 protein share the capacity to disrupt the interaction between transcription factor E2F and the retinoblastoma gene product. Proc. Natl. Acad. Sci. USA 894549-4553. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

