CD44 variant isoforms in head and neck squamous cell carcinoma progression
- PMID: 19507218
- PMCID: PMC2718060
- DOI: 10.1002/lary.20506
CD44 variant isoforms in head and neck squamous cell carcinoma progression
Abstract
Objectives/hypothesis: The CD44 family of receptors includes multiple variant isoforms, several of which have been linked to malignant properties including migration, invasion, and metastasis. The objective of this study was to investigate the role of the CD44 v3, v6, and v10 variant isoforms in head and neck squamous cell carcinoma (HNSCC) tumor progression behaviors.
Study design: Laboratory study involving cell cultures and clinical tissue specimens.
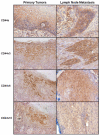
Methods: Analysis of the expression of standard CD44s and the CD44 variant isoforms v3, v6, and v10 was carried out in the HNSCC cell line, HSC-3. The role of CD44 isoforms in migration, proliferation, and cisplatin resistance was determined. Immunohistochemical analysis was performed on clinical tissue specimens obtained from a series of 82 HNSCC patients. The expression of standard CD44s and the CD44 v3, v6, and v10 variants in primary tumor specimens (n = 82) and metastatic cervical lymph nodes (n = 24) were analyzed with respect to various clinicopathologic variables.
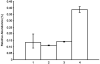
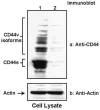
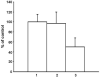
Results: HSC-3 cells express at least four CD44 isoforms, and these CD44 isoforms mediate migration, proliferation, and cisplatin sensitivity. Compared with primary tumors, a greater proportion of metastatic lymph nodes demonstrated strong expression of CD44 v3 (lymph node 14/24 vs. primary tumor 38/82), CD44 v6 (lymph node 18/24 vs. primary tumor 26/82), and CD44 v10 (lymph node 14/24 vs. primary tumor 16/82), while expression of standard CD44 was not significantly different in metastatic lymph nodes and primary tumors (lymph node 10/24 vs. primary tumor 60/82). Expression of CD44 variant isoforms were associated with advanced T stage (v3 and v6), regional (v3) and distant (v10) metastasis, perineural invasion (v6), and radiation failure (v10). CD44 v6 and CD44 v10 were also significantly associated with shorter disease-free survival.
Conclusions: CD44 isoforms mediate migration, proliferation, and cisplatin sensitivity in HNSCC. Furthermore, expression of certain CD44 variants may be important molecular markers for HNSCC progression and should be investigated as potential therapeutic targets for therapy.
Figures









Similar articles
-
Association of CD44 V3-containing isoforms with tumor cell growth, migration, matrix metalloproteinase expression, and lymph node metastasis in head and neck cancer.Head Neck. 2007 Jun;29(6):550-8. doi: 10.1002/hed.20544. Head Neck. 2007. PMID: 17252589
-
Significance of CD44 expression in head and neck cancer: a systemic review and meta-analysis.BMC Cancer. 2014 Jan 13;14:15. doi: 10.1186/1471-2407-14-15. BMC Cancer. 2014. PMID: 24410905 Free PMC article. Review.
-
Characterization of CD44v6 isoforms in head-and-neck squamous-cell carcinoma.Int J Cancer. 1999 Sep 9;82(6):837-45. doi: 10.1002/(sici)1097-0215(19990909)82:6<837::aid-ijc12>3.0.co;2-h. Int J Cancer. 1999. PMID: 10446451
-
Role of hyaluronan-mediated CD44 signaling in head and neck squamous cell carcinoma progression and chemoresistance.Am J Pathol. 2011 Mar;178(3):956-63. doi: 10.1016/j.ajpath.2010.11.077. Am J Pathol. 2011. PMID: 21356346 Free PMC article. Review.
-
Evaluation of CD44 variant expression in oral, head and neck squamous cell carcinomas using a triple approach and its clinical significance.Int J Immunopathol Pharmacol. 2014 Jul-Sep;27(3):337-49. doi: 10.1177/039463201402700304. Int J Immunopathol Pharmacol. 2014. PMID: 25280025 Free PMC article.
Cited by
-
Cancer stem cells in head and neck cancer.Onco Targets Ther. 2012;5:375-83. doi: 10.2147/OTT.S38694. Epub 2012 Nov 21. Onco Targets Ther. 2012. PMID: 23189032 Free PMC article.
-
Nuclear BMI-1 expression in laryngeal carcinoma correlates with lymph node pathological status.World J Surg Oncol. 2012 Oct 2;10:206. doi: 10.1186/1477-7819-10-206. World J Surg Oncol. 2012. PMID: 23031716 Free PMC article.
-
More than markers: biological significance of cancer stem cell-defining molecules.Mol Cancer Ther. 2010 Sep;9(9):2450-7. doi: 10.1158/1535-7163.MCT-10-0530. Epub 2010 Aug 17. Mol Cancer Ther. 2010. PMID: 20716638 Free PMC article. Review.
-
Cancer stem cells in head and neck squamous cell carcinoma.J Oncol. 2011;2011:762780. doi: 10.1155/2011/762780. Epub 2010 Nov 8. J Oncol. 2011. PMID: 21076545 Free PMC article.
-
Cancer Stem Cells in Head and Neck Squamous Cell Carcinoma: Identification, Characterization and Clinical Implications.Cancers (Basel). 2019 May 2;11(5):616. doi: 10.3390/cancers11050616. Cancers (Basel). 2019. PMID: 31052565 Free PMC article. Review.
References
-
- Parkin DM, Pisani P, Ferlay J. Global cancer statistics, 2002. Ca Cancer J Clin. 2005;55:74–108. - PubMed
-
- Turley E, Noble P, Bourguignon L. Signaling properties of hyaluronan receptors. J Biol Chem. 2002;277:4589–92. - PubMed
-
- Bourguignon LY, Singleton PA, Diedrich F, et al. CD44 interaction with Na+-H+ exchanger (NHE1) creates acidic microenvironments leading to hyaluronidase-2 and cathepsin B activation and breast tumor cell invasion. J Biol Chem. 2004;279:26991–7007. - PubMed
-
- Bourguignon LY, Zhu H, Zhou B, et al. Hyaluronan promotes CD44v3-Vav2 interaction with Grb2-p185HER2 and induces Rac1 and Ras signaling during ovarian tumor cell migration and growth. J Biol Chem. 2001;276:48679–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

