Gamma-actin is required for cytoskeletal maintenance but not development
- PMID: 19497859
- PMCID: PMC2701000
- DOI: 10.1073/pnas.0900221106
Gamma-actin is required for cytoskeletal maintenance but not development
Abstract
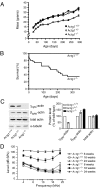
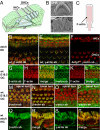
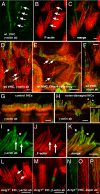
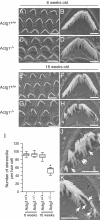
Beta(cyto)-actin and gamma(cyto)-actin are ubiquitous proteins thought to be essential building blocks of the cytoskeleton in all non-muscle cells. Despite this widely held supposition, we show that gamma(cyto)-actin null mice (Actg1(-/-)) are viable. However, they suffer increased mortality and show progressive hearing loss during adulthood despite compensatory up-regulation of beta(cyto)-actin. The surprising viability and normal hearing of young Actg1(-/-) mice means that beta(cyto)-actin can likely build all essential non-muscle actin-based cytoskeletal structures including mechanosensory stereocilia of hair cells that are necessary for hearing. Although gamma(cyto)-actin-deficient stereocilia form normally, we found that they cannot maintain the integrity of the stereocilia actin core. In the wild-type, gamma(cyto)-actin localizes along the length of stereocilia but re-distributes to sites of F-actin core disruptions resulting from animal exposure to damaging noise. In Actg1(-/-) stereocilia similar disruptions are observed even without noise exposure. We conclude that gamma(cyto)-actin is required for reinforcement and long-term stability of F-actin-based structures but is not an essential building block of the developing cytoskeleton.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Stereocilia morphogenesis and maintenance through regulation of actin stability.Semin Cell Dev Biol. 2017 May;65:88-95. doi: 10.1016/j.semcdb.2016.08.017. Epub 2016 Aug 23. Semin Cell Dev Biol. 2017. PMID: 27565685 Free PMC article. Review.
-
β-actin and γ-actin are each dispensable for auditory hair cell development but required for Stereocilia maintenance.PLoS Genet. 2010 Oct 14;6(10):e1001158. doi: 10.1371/journal.pgen.1001158. PLoS Genet. 2010. PMID: 20976199 Free PMC article.
-
Delayed embryonic development and impaired cell growth and survival in Actg1 null mice.Cytoskeleton (Hoboken). 2010 Sep;67(9):564-72. doi: 10.1002/cm.20467. Cytoskeleton (Hoboken). 2010. PMID: 20662086 Free PMC article.
-
Cytoplasmic gamma-actin is not required for skeletal muscle development but its absence leads to a progressive myopathy.Dev Cell. 2006 Sep;11(3):387-97. doi: 10.1016/j.devcel.2006.07.001. Dev Cell. 2006. PMID: 16950128
-
Building and repairing the stereocilia cytoskeleton in mammalian auditory hair cells.Hear Res. 2019 May;376:47-57. doi: 10.1016/j.heares.2018.12.012. Epub 2019 Jan 2. Hear Res. 2019. PMID: 30638948 Free PMC article. Review.
Cited by
-
Severe forms of Baraitser-Winter syndrome are caused by ACTB mutations rather than ACTG1 mutations.Eur J Hum Genet. 2014 Feb;22(2):179-83. doi: 10.1038/ejhg.2013.130. Epub 2013 Jun 12. Eur J Hum Genet. 2014. PMID: 23756437 Free PMC article.
-
Stereocilia morphogenesis and maintenance through regulation of actin stability.Semin Cell Dev Biol. 2017 May;65:88-95. doi: 10.1016/j.semcdb.2016.08.017. Epub 2016 Aug 23. Semin Cell Dev Biol. 2017. PMID: 27565685 Free PMC article. Review.
-
The remodelling of actin composition as a hallmark of cancer.Transl Oncol. 2021 Jun;14(6):101051. doi: 10.1016/j.tranon.2021.101051. Epub 2021 Mar 21. Transl Oncol. 2021. PMID: 33761369 Free PMC article. Review.
-
A corticosteroid-responsive transcription factor, promyelocytic leukemia zinc finger protein, mediates protection of the cochlea from acoustic trauma.J Neurosci. 2011 Jan 12;31(2):735-41. doi: 10.1523/JNEUROSCI.3955-10.2011. J Neurosci. 2011. PMID: 21228182 Free PMC article.
-
Roles of the espin actin-bundling proteins in the morphogenesis and stabilization of hair cell stereocilia revealed in CBA/CaJ congenic jerker mice.PLoS Genet. 2011 Mar;7(3):e1002032. doi: 10.1371/journal.pgen.1002032. Epub 2011 Mar 24. PLoS Genet. 2011. PMID: 21455486 Free PMC article.
References
-
- Herman IM. Actin isoforms. Curr Opin Cell Biol. 1993;5:48–55. - PubMed
-
- Furness DN, Katori Y, Mahendrasingam S, Hackney CM. Differential distribution of beta- and gamma-actin in guinea-pig cochlear sensory and supporting cells. Hear Res. 2005;207:22–34. - PubMed
-
- Hofer D, Ness W, Drenckhahn D. Sorting of actin isoforms in chicken auditory hair cells. J Cell Sci. 1997;110:765–770. - PubMed
-
- Slepecky NB, Savage JE. Expression of actin isoforms in the guinea pig organ of Corti: Muscle isoforms are not detected. Hear Res. 1994;73:16–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

