A genome-wide RNAi screen identifies multiple synthetic lethal interactions with the Ras oncogene
- PMID: 19490893
- PMCID: PMC2768667
- DOI: 10.1016/j.cell.2009.05.006
A genome-wide RNAi screen identifies multiple synthetic lethal interactions with the Ras oncogene
Abstract
Oncogenic mutations in the small GTPase Ras are highly prevalent in cancer, but an understanding of the vulnerabilities of these cancers is lacking. We undertook a genome-wide RNAi screen to identify synthetic lethal interactions with the KRAS oncogene. We discovered a diverse set of proteins whose depletion selectively impaired the viability of Ras mutant cells. Among these we observed a strong enrichment for genes with mitotic functions. We describe a pathway involving the mitotic kinase PLK1, the anaphase-promoting complex/cyclosome, and the proteasome that, when inhibited, results in prometaphase accumulation and the subsequent death of Ras mutant cells. Gene expression analysis indicates that reduced expression of genes in this pathway correlates with increased survival of patients bearing tumors with a Ras transcriptional signature. Our results suggest a previously underappreciated role for Ras in mitotic progression and demonstrate a pharmacologically tractable pathway for the potential treatment of cancers harboring Ras mutations.
Figures
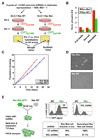
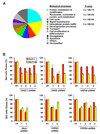
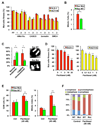
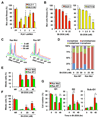
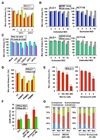
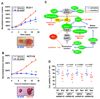
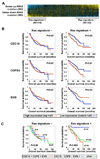
Comment in
-
Finding and drugging the vulnerabilities of RAS-dependent cancers.Cell. 2009 May 29;137(5):796-8. doi: 10.1016/j.cell.2009.05.011. Cell. 2009. PMID: 19490885
-
shRNA technology: investigating Ras-dependent cancer.Cancer Biol Ther. 2009 Oct;8(19):1798-9. doi: 10.4161/cbt.8.19.9681. Cancer Biol Ther. 2009. PMID: 19713764 No abstract available.
Similar articles
-
Determination of synthetic lethal interactions in KRAS oncogene-dependent cancer cells reveals novel therapeutic targeting strategies.Cell Res. 2012 Aug;22(8):1227-45. doi: 10.1038/cr.2012.82. Epub 2012 May 22. Cell Res. 2012. PMID: 22613949 Free PMC article.
-
Synthetic lethal interaction between oncogenic KRAS dependency and STK33 suppression in human cancer cells.Cell. 2009 May 29;137(5):821-34. doi: 10.1016/j.cell.2009.03.017. Cell. 2009. PMID: 19490892
-
The PLK1 inhibitor GSK461364A is effective in poorly differentiated and anaplastic thyroid carcinoma cells, independent of the nature of their driver mutations.Thyroid. 2013 Oct;23(10):1284-93. doi: 10.1089/thy.2013.0037. Epub 2013 Jul 25. Thyroid. 2013. PMID: 23509868 Free PMC article.
-
Targeting mutant KRAS for anticancer therapeutics: a review of novel small molecule modulators.J Med Chem. 2013 Jul 11;56(13):5219-30. doi: 10.1021/jm3017706. Epub 2013 Apr 23. J Med Chem. 2013. PMID: 23566315 Free PMC article. Review.
-
Targeting polo-like kinase 1 for cancer therapy.Nat Rev Cancer. 2006 Apr;6(4):321-30. doi: 10.1038/nrc1841. Nat Rev Cancer. 2006. PMID: 16557283 Review.
Cited by
-
Essential role of Cdc42 in Ras-induced transformation revealed by gene targeting.PLoS One. 2012;7(6):e37317. doi: 10.1371/journal.pone.0037317. Epub 2012 Jun 18. PLoS One. 2012. PMID: 22719838 Free PMC article.
-
Real-time single-molecule co-immunoprecipitation analyses reveal cancer-specific Ras signalling dynamics.Nat Commun. 2013;4:1505. doi: 10.1038/ncomms2507. Nat Commun. 2013. PMID: 23422673 Free PMC article.
-
Therapeutic Approaches to RAS Mutation.Cancer J. 2016 May-Jun;22(3):165-74. doi: 10.1097/PPO.0000000000000187. Cancer J. 2016. PMID: 27341593 Free PMC article. Review.
-
Oncoprotein-specific molecular interaction maps (SigMaps) for cancer network analyses.Nat Biotechnol. 2021 Feb;39(2):215-224. doi: 10.1038/s41587-020-0652-7. Epub 2020 Sep 14. Nat Biotechnol. 2021. PMID: 32929263 Free PMC article.
-
Selective requirement for Mediator MED23 in Ras-active lung cancer.Proc Natl Acad Sci U S A. 2012 Oct 9;109(41):E2813-22. doi: 10.1073/pnas.1204311109. Epub 2012 Sep 17. Proc Natl Acad Sci U S A. 2012. PMID: 22988093 Free PMC article.
References
-
- Barr FA, Sillje HH, Nigg EA. Polo-like kinases and the orchestration of cell division. Nat Rev Mol Cell Biol. 2004;5:429–440. - PubMed
-
- Bezieau S, Devilder MC, Avet-Loiseau H, Mellerin MP, Puthier D, Pennarun E, Rapp MJ, Harousseau JL, Moisan JP, Bataille R. High incidence of N and K-Ras activating mutations in multiple myeloma and primary plasma cell leukemia at diagnosis. Hum Mutat. 2001;18:212–224. - PubMed
-
- Cope GA, Deshaies RJ. COP9 signalosome: a multifunctional regulator of SCF and other cullin-based ubiquitin ligases. Cell. 2003;114:663–671. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

