Discrimination of common and unique RNA-binding activities among Fragile X mental retardation protein paralogs
- PMID: 19487368
- PMCID: PMC2722981
- DOI: 10.1093/hmg/ddp255
Discrimination of common and unique RNA-binding activities among Fragile X mental retardation protein paralogs
Abstract
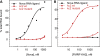
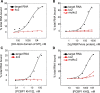
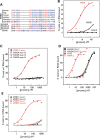
Fragile X mental retardation is caused by loss-of-function of a single gene encoding FMRP, an RNA-binding protein that harbors three canonical RNA-binding domains, two KH-type and one RGG box. Two autosomal paralogs of FMRP, FXR1P and FXR2P, are similar to FMRP in their overall structure, including the presence of putative RNA-binding domains, but to what extent they provide functional redundancy with FMRP is unclear. Although FMRP has been characterized as a polyribosome-associated regulator of translation, less is known about the functions of FXR1P and FXR2P. For example, FMRP binds intramolecular G-quadruplex and kissing complex RNA (kcRNA) ligands via the RGG box and KH2 domain, respectively, although the RNA ligands of FXR1P and FXR2P are unknown. Here we demonstrate that FXR1P and FXR2P KH2 domains bind kcRNA ligands with the same affinity as the FMRP KH2 domain although other KH domains do not. RNA ligand recognition by this family is highly conserved, as the KH2 domain of the single Drosophila ortholog, dFMRP, also binds kcRNA. kcRNA was able to displace FXR1P and FXR2P from polyribosomes as it does for FMRP, and this displacement was FMRP-independent. This suggests that all three family members recognize the same binding site on RNA mediating their polyribosome association, and that they may be functionally redundant with regard to this aspect of translational control. In contrast, FMRP is unique in its ability to recognize G-quadruplexes, suggesting the FMRP RGG domain may play a non-redundant role in the pathophysiology of the disease.
Figures





Similar articles
-
Kissing complex RNAs mediate interaction between the Fragile-X mental retardation protein KH2 domain and brain polyribosomes.Genes Dev. 2005 Apr 15;19(8):903-18. doi: 10.1101/gad.1276805. Epub 2005 Apr 1. Genes Dev. 2005. PMID: 15805463 Free PMC article.
-
RNA-Binding Specificity of the Human Fragile X Mental Retardation Protein.J Mol Biol. 2020 Jun 12;432(13):3851-3868. doi: 10.1016/j.jmb.2020.04.021. Epub 2020 Apr 25. J Mol Biol. 2020. PMID: 32343993 Free PMC article.
-
Interactions of the G quartet forming semaphorin 3F RNA with the RGG box domain of the fragile X protein family.Nucleic Acids Res. 2007;35(16):5379-92. doi: 10.1093/nar/gkm581. Epub 2007 Aug 9. Nucleic Acids Res. 2007. PMID: 17693432 Free PMC article.
-
FMRP RNA targets: identification and validation.Genes Brain Behav. 2005 Aug;4(6):341-9. doi: 10.1111/j.1601-183X.2005.00144.x. Genes Brain Behav. 2005. PMID: 16098133 Review.
-
Biology of the fragile X mental retardation protein, an RNA-binding protein.Biochem Cell Biol. 1999;77(4):331-42. Biochem Cell Biol. 1999. PMID: 10546896 Review.
Cited by
-
Localization and local translation of Arc/Arg3.1 mRNA at synapses: some observations and paradoxes.Front Mol Neurosci. 2015 Jan 12;7:101. doi: 10.3389/fnmol.2014.00101. eCollection 2014. Front Mol Neurosci. 2015. PMID: 25628532 Free PMC article.
-
Spatial control of nucleoporin condensation by fragile X-related proteins.EMBO J. 2020 Oct 15;39(20):e104467. doi: 10.15252/embj.2020104467. Epub 2020 Jul 24. EMBO J. 2020. PMID: 32706158 Free PMC article.
-
Memory deficits, gait ataxia and neuronal loss in the hippocampus and cerebellum in mice that are heterozygous for Pur-alpha.Neuroscience. 2016 Nov 19;337:177-190. doi: 10.1016/j.neuroscience.2016.09.018. Epub 2016 Sep 17. Neuroscience. 2016. PMID: 27651147 Free PMC article.
-
The pur protein family: genetic and structural features in development and disease.J Cell Physiol. 2013 May;228(5):930-7. doi: 10.1002/jcp.24237. J Cell Physiol. 2013. PMID: 23018800 Free PMC article. Review.
-
A common molecular mechanism underlies two phenotypically distinct 17p13.1 microdeletion syndromes.Am J Hum Genet. 2010 Nov 12;87(5):631-42. doi: 10.1016/j.ajhg.2010.10.007. Am J Hum Genet. 2010. PMID: 21056402 Free PMC article.
References
-
- Gibson T.J., Rice P.M., Thompson J.D., Heringa J. KH domains within the FMR1 sequence suggest that fragile X syndrome stems from a defect in RNA metabolism. Trends Biochem. Sci. 1993;18:331–333. - PubMed
-
- Ashley C.T., Wilkinson K.D., Reines D., Warren S.T. FMR-1 protein: conserved RNP family domains and selective RNA binding. Science. 1993;262:563–566. - PubMed
-
- Siomi H., Siomi M.C., Nussbaum R.L., Dreyfuss G. The protein product of the fragile X gene, FMR1, has characteristics of an RNA-binding protein. Cell. 1993;74:291–298. - PubMed
-
- DeBoulle K., Verkerk A.J., Reyniers E., Vits L., Hendrickx J., Van Roy B., Van Den Bos F., de Graaff E., Oostra B.A., Willems P.J. A point mutation in the FMR-1 gene associated with fragile X mental retardation. Nat. Genet. 1993;3:31–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

