Notch signaling is required for lateral induction of Jagged1 during FGF-induced lens fiber differentiation
- PMID: 19481073
- PMCID: PMC2730671
- DOI: 10.1016/j.ydbio.2009.05.566
Notch signaling is required for lateral induction of Jagged1 during FGF-induced lens fiber differentiation
Abstract
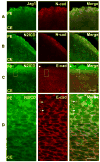
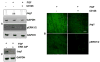
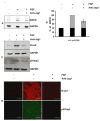
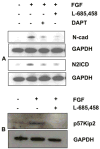
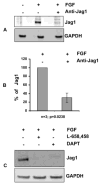
Previous studies of the developing lens have shown that Notch signaling regulates differentiation of lens fiber cells by maintaining a proliferating precursor pool in the anterior epithelium. However, whether Notch signaling is further required after the onset of fiber cell differentiation is not clear. This work investigates the role of Notch2 and Jagged1 (Jag1) in secondary fiber cell differentiation using rat lens epithelial explants undergoing FGF-2 dependent differentiation in vitro. FGF induced Jag1 expression and Notch2 signaling (as judged by the appearance of activated Notch2 Intracellular Domain (N2ICD)) within 12-24 h. These changes were correlated with induction of the Notch effector, Hes5, upregulation of N-cadherin (N-cad), and downregulation of E-cadherin (E-cad), a cadherin switch characteristic of fiber cell differentiation. Induction of Jag1 was efficiently blocked by U0126, a specific inhibitor of MAPK/ERK signaling, indicating a requirement for signaling through this pathway downstream of the FGF receptor. Other growth factors that activate MAPK/ERK signaling (EGF, PDGF, IGF) did not induce Jag1. Inhibition of Notch signaling using gamma secretase inhibitors DAPT and L-685,458 or anti-Jag1 antibody markedly decreased FGF-dependent expression of Jag1 demonstrating Notch-dependent lateral induction. In addition, inhibition of Notch signaling reduced expression of N-cad, and the cyclin dependent kinase inhibitor, p57Kip2, indicating a direct role for Notch signaling in secondary fiber cell differentiation. These results demonstrate that Notch-mediated lateral induction of Jag1 is an essential component of FGF-dependent lens fiber cell differentiation.
Figures



Similar articles
-
PKCδ is required for Jagged-1 induction of human mesenchymal stem cell osteogenic differentiation.Stem Cells. 2013 Jun;31(6):1181-92. doi: 10.1002/stem.1353. Stem Cells. 2013. PMID: 23404789
-
Ligand-dependent Notch signaling strength orchestrates lateral induction and lateral inhibition in the developing inner ear.Development. 2014 Jun;141(11):2313-24. doi: 10.1242/dev.108100. Epub 2014 May 12. Development. 2014. PMID: 24821984
-
Interactions between lens epithelial and fiber cells reveal an intrinsic self-assembly mechanism.Dev Biol. 2014 Jan 15;385(2):291-303. doi: 10.1016/j.ydbio.2013.10.030. Epub 2013 Nov 8. Dev Biol. 2014. PMID: 24211762 Free PMC article.
-
Blockade of Jagged/Notch pathway abrogates transforming growth factor β2-induced epithelial-mesenchymal transition in human retinal pigment epithelium cells.Curr Mol Med. 2014 May;14(4):523-34. doi: 10.2174/1566524014666140331230411. Curr Mol Med. 2014. PMID: 24694299 Review.
-
Anti-Jagged-1 immunotherapy in cancer.Adv Med Sci. 2022 Sep;67(2):196-202. doi: 10.1016/j.advms.2022.04.001. Epub 2022 Apr 11. Adv Med Sci. 2022. PMID: 35421813 Review.
Cited by
-
The cellular Notch1 protein promotes KSHV reactivation in an Rta-dependent manner.J Virol. 2024 Aug 20;98(8):e0078824. doi: 10.1128/jvi.00788-24. Epub 2024 Jul 8. J Virol. 2024. PMID: 38975769 Free PMC article.
-
Notch signaling influences neuroprotective and proliferative properties of mature Müller glia.J Neurosci. 2010 Feb 24;30(8):3101-12. doi: 10.1523/JNEUROSCI.4919-09.2010. J Neurosci. 2010. PMID: 20181607 Free PMC article.
-
Signaling mechanisms controlling cranial placode neurogenesis and delamination.Dev Biol. 2014 May 1;389(1):39-49. doi: 10.1016/j.ydbio.2013.11.025. Epub 2013 Dec 3. Dev Biol. 2014. PMID: 24315854 Free PMC article. Review.
-
Wnt-frizzled signaling is part of an FGF-induced cascade that promotes lens fiber differentiation.Invest Ophthalmol Vis Sci. 2013 Mar 1;54(3):1582-90. doi: 10.1167/iovs.12-11357. Invest Ophthalmol Vis Sci. 2013. PMID: 23385791 Free PMC article.
-
The lens in focus: a comparison of lens development in Drosophila and vertebrates.Mol Genet Genomics. 2011 Oct;286(3-4):189-213. doi: 10.1007/s00438-011-0643-y. Epub 2011 Aug 30. Mol Genet Genomics. 2011. PMID: 21877135 Free PMC article. Review.
References
-
- Ang SJ, Stump RJ, Lovicu FJ, McAvoy JW. Spatial and temporal expression of Wnt and Dickkopf genes during murine lens development. Gene Expr Patterns. 2004;4:289–95. - PubMed
-
- Bray S. Notch signalling in Drosophila: three ways to use a pathway. Semin Cell Dev Biol. 1998;9:591–7. - PubMed
-
- Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–89. - PubMed
-
- Cain S, Martinez G, Kokkinos MI, Turner K, Richardson RJ, Abud HE, Huelsken J, Robinson ML, de Iongh RU. Differential requirement for beta-catenin in epithelial and fiber cells during lens development. Dev Biol. 2008;321:420–33. - PubMed
-
- Chandrasekher G, Sailaja D. Differential activation of phosphatidylinositol 3-kinase signaling during proliferation and differentiation of lens epithelial cells. Invest Ophthalmol Vis Sci. 2003;44:4400–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

