Induction of HoxB transcription by retinoic acid requires actin polymerization
- PMID: 19477923
- PMCID: PMC2719572
- DOI: 10.1091/mbc.e09-02-0114
Induction of HoxB transcription by retinoic acid requires actin polymerization
Abstract
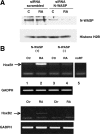
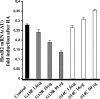
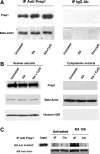
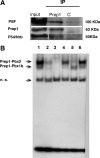
We have analyzed the role of actin polymerization in retinoic acid (RA)-induced HoxB transcription, which is mediated by the HoxB regulator Prep1. RA induction of the HoxB genes can be prevented by the inhibition of actin polymerization. Importantly, inhibition of actin polymerization specifically affects the transcription of inducible Hox genes, but not that of their transcriptional regulators, the RARs, nor of constitutively expressed, nor of actively transcribed Hox genes. RA treatment induces the recruitment to the HoxB2 gene enhancer of a complex composed of "elongating" RNAPII, Prep1, beta-actin, and N-WASP as well as the accessory splicing components p54Nrb and PSF. We show that inhibition of actin polymerization prevents such recruitment. We conclude that inducible Hox genes are selectively sensitive to the inhibition of actin polymerization and that actin polymerization is required for the assembly of a transcription complex on the regulatory region of the Hox genes.
Figures
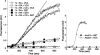
Similar articles
-
Co-transcriptional nuclear actin dynamics.Nucleus. 2013 Jan-Feb;4(1):43-52. doi: 10.4161/nucl.22798. Epub 2012 Nov 8. Nucleus. 2013. PMID: 23138849 Free PMC article. Review.
-
Regulation of RNA-polymerase-II-dependent transcription by N-WASP and its nuclear-binding partners.Nat Cell Biol. 2006 Jul;8(7):756-63. doi: 10.1038/ncb1433. Epub 2006 Jun 11. Nat Cell Biol. 2006. PMID: 16767080
-
Nuclear actin polymerization from faster growing ends in the initial activation of Hox gene transcription are nuclear speckles involved?Transcription. 2013 Sep-Dec;4(5):260-72. doi: 10.4161/trns.27672. Transcription. 2013. PMID: 24406343
-
Retinoid regulated association of transcriptional co-regulators and the polycomb group protein SUZ12 with the retinoic acid response elements of Hoxa1, RARbeta(2), and Cyp26A1 in F9 embryonal carcinoma cells.J Mol Biol. 2007 Sep 14;372(2):298-316. doi: 10.1016/j.jmb.2007.06.079. Epub 2007 Jul 3. J Mol Biol. 2007. PMID: 17663992 Free PMC article.
-
Paraspeckles: nuclear bodies built on long noncoding RNA.J Cell Biol. 2009 Sep 7;186(5):637-44. doi: 10.1083/jcb.200906113. Epub 2009 Aug 31. J Cell Biol. 2009. PMID: 19720872 Free PMC article. Review.
Cited by
-
PSF: nuclear busy-body or nuclear facilitator?Wiley Interdiscip Rev RNA. 2015 Jul-Aug;6(4):351-67. doi: 10.1002/wrna.1280. Epub 2015 Apr 1. Wiley Interdiscip Rev RNA. 2015. PMID: 25832716 Free PMC article. Review.
-
Co-transcriptional nuclear actin dynamics.Nucleus. 2013 Jan-Feb;4(1):43-52. doi: 10.4161/nucl.22798. Epub 2012 Nov 8. Nucleus. 2013. PMID: 23138849 Free PMC article. Review.
-
Transcription factor binding at enhancers: shaping a genomic regulatory landscape in flux.Front Genet. 2012 Sep 28;3:195. doi: 10.3389/fgene.2012.00195. eCollection 2012. Front Genet. 2012. PMID: 23060900 Free PMC article.
-
Involvement of the SATB1/F-actin complex in chromatin reorganization during active cell death.Int J Mol Med. 2014 Jun;33(6):1441-50. doi: 10.3892/ijmm.2014.1710. Epub 2014 Mar 21. Int J Mol Med. 2014. PMID: 24676287 Free PMC article.
-
Nuclear actin polymerization is required for transcriptional reprogramming of Oct4 by oocytes.Genes Dev. 2011 May 1;25(9):946-58. doi: 10.1101/gad.615211. Genes Dev. 2011. PMID: 21536734 Free PMC article.
References
-
- Bettinger B. T., Gilbert D. M., Amberg D. C. Actin up in the nucleus. Nat. Rev. Mol. Cell. Biol. 2004;5:410–415. - PubMed
-
- Chuang C. H., Carpenter A. E., Fuchsova B., Johnson T., de Lanerolle P., Belmont A. S. Long-range directional movement of an interphase chromosome site. Curr. Biol. 2006;16:825–831. - PubMed
-
- Deflorian G., Tiso N., Ferretti E., Meyer D., Blasi F., Bortolussi M., Argenton F. Prep1.1 has essential genetic functions in hindbrain development and cranial neural crest cell differentiation. Development. 2004;131:613–627. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

