Inhibitory regulation of electrically coupled neurons in the inferior olive is mediated by asynchronous release of GABA
- PMID: 19477156
- PMCID: PMC3261724
- DOI: 10.1016/j.neuron.2009.04.018
Inhibitory regulation of electrically coupled neurons in the inferior olive is mediated by asynchronous release of GABA
Abstract
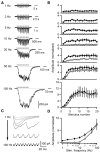
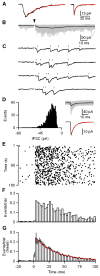
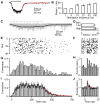
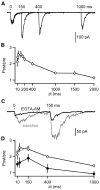
Inhibitory projection neurons in the deep cerebellar nuclei (DCN) provide GABAergic input to neurons of the inferior olive (IO) that in turn produce climbing fiber synapses onto Purkinje cells. Anatomical evidence suggests that DCN to IO synapses control electrical coupling between IO neurons. In vivo studies suggest that they also control the synchrony of IO neurons and play an important role in cerebellar learning. Here we describe the DCN to IO synapse. Remarkably, GABA release was almost exclusively asynchronous, with little conventional synchronous release. Synaptic transmission was extremely frequency dependent, with low-frequency stimulation being largely ineffective. However, due to the prominence of asynchronous release, stimulation at frequencies above 10 Hz evoked steady-state inhibitory currents. These properties seem ideally suited to transform the firing rate of DCN neurons into sustained inhibition that both suppresses the firing of IO cells and regulates the effective coupling between IO neurons.
Figures

Similar articles
-
Modulatory effects of serotonin on GABAergic synaptic transmission and membrane properties in the deep cerebellar nuclei.J Neurophysiol. 2009 Mar;101(3):1361-74. doi: 10.1152/jn.90750.2008. Epub 2009 Jan 14. J Neurophysiol. 2009. PMID: 19144744
-
Cerebellar and vestibular nuclear synapses in the inferior olive have distinct release kinetics and neurotransmitters.Elife. 2020 Dec 1;9:e61672. doi: 10.7554/eLife.61672. Elife. 2020. PMID: 33259288 Free PMC article.
-
GABAergic modulation of complex spike activity by the cerebellar nucleoolivary pathway in rat.J Neurophysiol. 1996 Jul;76(1):255-75. doi: 10.1152/jn.1996.76.1.255. J Neurophysiol. 1996. PMID: 8836223
-
Increased Purkinje Cell Complex Spike and Deep Cerebellar Nucleus Synchrony as a Potential Basis for Syndromic Essential Tremor. A Review and Synthesis of the Literature.Cerebellum. 2021 Apr;20(2):266-281. doi: 10.1007/s12311-020-01197-5. Epub 2020 Oct 13. Cerebellum. 2021. PMID: 33048308 Review.
-
GABA-ergic transmission in deep cerebellar nuclei.Prog Neurobiol. 1997 Oct;53(2):259-71. doi: 10.1016/s0301-0082(97)00033-6. Prog Neurobiol. 1997. PMID: 9364613 Review.
Cited by
-
Electrical synapses and their functional interactions with chemical synapses.Nat Rev Neurosci. 2014 Apr;15(4):250-63. doi: 10.1038/nrn3708. Epub 2014 Mar 12. Nat Rev Neurosci. 2014. PMID: 24619342 Free PMC article. Review.
-
An alien divalent ion reveals a major role for Ca²⁺ buffering in controlling slow transmitter release.J Neurosci. 2014 Sep 17;34(38):12622-35. doi: 10.1523/JNEUROSCI.1990-14.2014. J Neurosci. 2014. PMID: 25232102 Free PMC article.
-
The Contribution of Brainstem and Cerebellar Pathways to Auditory Recognition.Front Psychol. 2017 Mar 20;8:265. doi: 10.3389/fpsyg.2017.00265. eCollection 2017. Front Psychol. 2017. PMID: 28373850 Free PMC article. Review.
-
The Mechanisms and Functions of Synaptic Facilitation.Neuron. 2017 May 3;94(3):447-464. doi: 10.1016/j.neuron.2017.02.047. Neuron. 2017. PMID: 28472650 Free PMC article. Review.
-
Hypertrophy of the inferior olivary nucleus impacts perception of gravity.Front Neurol. 2012 May 11;3:79. doi: 10.3389/fneur.2012.00079. eCollection 2012. Front Neurol. 2012. PMID: 22593754 Free PMC article.
References
-
- Alley K, Baker R, Simpson JI. Afferents to the vestibulo-cerebellum and the origin of the visual climbing fibers in the rabbit. Brain Res. 1975;98:582–589. - PubMed
-
- Bal T, McCormick DA. Synchronized oscillations in the inferior olive are controlled by the hyperpolarization-activated cation current I(h) J Neurophysiol. 1997;77:3145–3156. - PubMed
-
- Balaban CD, Beryozkin G. Organization of vestibular nucleus projections to the caudal dorsal cap of kooy in rabbits. Neuroscience. 1994;62:1217–1236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

