Plk1-mediated phosphorylation of Topors regulates p53 stability
- PMID: 19473992
- PMCID: PMC2707202
- DOI: 10.1074/jbc.C109.001560
Plk1-mediated phosphorylation of Topors regulates p53 stability
Abstract
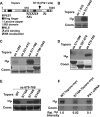
Polo-like kinase 1 (Plk1) overexpression is associated with tumorigenesis by an unknown mechanism. Likewise, Plk1 was suggested to act as a negative regulator of tumor suppressor p53, but the mechanism remains to be determined. Herein, we have identified topoisomerase I-binding protein (Topors), a p53-binding protein, as a Plk1 target. We show that Plk1 phosphorylates Topors on Ser(718) in vivo. Significantly, expression of a Plk1-unphosphorylatable Topors mutant (S718A) leads to a dramatic accumulation of p53 through inhibition of p53 degradation. Topors is an ubiquitin and small ubiquitin-like modifier ubiquitin-protein isopeptide ligase (SUMO E3) ligase. Plk1-mediated phosphorylation of Topors inhibits Topors-mediated sumoylation of p53, whereas p53 ubiquitination is enhanced, leading to p53 degradation. These results demonstrate that Plk1 modulates Topors activity in suppressing p53 function and identify a likely mechanism for the tumorigenic potential of Plk1.
Figures

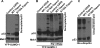
Similar articles
-
Plk1 phosphorylation of Topors is involved in its degradation.Mol Biol Rep. 2010 Jul;37(6):3023-8. doi: 10.1007/s11033-009-9871-1. Epub 2009 Oct 11. Mol Biol Rep. 2010. PMID: 19821153 Free PMC article.
-
Identification of phosphorylation sites of TOPORS and a role for serine 98 in the regulation of ubiquitin but not SUMO E3 ligase activity.Biochemistry. 2008 Dec 30;47(52):13887-96. doi: 10.1021/bi801904q. Biochemistry. 2008. PMID: 19053840
-
Topors acts as a SUMO-1 E3 ligase for p53 in vitro and in vivo.FEBS Lett. 2005 Sep 12;579(22):5007-12. doi: 10.1016/j.febslet.2005.07.088. FEBS Lett. 2005. PMID: 16122737
-
The substrates of Plk1, beyond the functions in mitosis.Protein Cell. 2010 Nov;1(11):999-1010. doi: 10.1007/s13238-010-0131-x. Epub 2010 Dec 10. Protein Cell. 2010. PMID: 21153517 Free PMC article. Review.
-
p53 regulation: teamwork between RING domains of Mdm2 and MdmX.Cell Cycle. 2011 Dec 15;10(24):4225-9. doi: 10.4161/cc.10.24.18662. Epub 2011 Dec 15. Cell Cycle. 2011. PMID: 22134240 Review.
Cited by
-
DNA damage-induced activation of ATM promotes β-TRCP-mediated Mdm2 ubiquitination and destruction.Oncotarget. 2012 Sep;3(9):1026-35. doi: 10.18632/oncotarget.640. Oncotarget. 2012. PMID: 22976441 Free PMC article.
-
Inhibition of Polo-like kinase 1 during the DNA damage response is mediated through loss of Aurora A recruitment by Bora.Oncogene. 2017 Mar 30;36(13):1840-1848. doi: 10.1038/onc.2016.347. Epub 2016 Oct 10. Oncogene. 2017. PMID: 27721411 Free PMC article.
-
Small molecule inhibition of polo-like kinase 1 by volasertib (BI 6727) causes significant melanoma growth delay and regression in vivo.Cancer Lett. 2017 Jan 28;385:179-187. doi: 10.1016/j.canlet.2016.10.025. Epub 2016 Oct 25. Cancer Lett. 2017. PMID: 27793694 Free PMC article.
-
Regulating tumor suppressor genes: post-translational modifications.Signal Transduct Target Ther. 2020 Jun 10;5(1):90. doi: 10.1038/s41392-020-0196-9. Signal Transduct Target Ther. 2020. PMID: 32532965 Free PMC article. Review.
-
Polo-like kinase 1 inhibitors, mitotic stress and the tumor suppressor p53.Cell Cycle. 2013 May 1;12(9):1340-51. doi: 10.4161/cc.24573. Epub 2013 Apr 10. Cell Cycle. 2013. PMID: 23574746 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

