JAK mutations in high-risk childhood acute lymphoblastic leukemia
- PMID: 19470474
- PMCID: PMC2695045
- DOI: 10.1073/pnas.0811761106
JAK mutations in high-risk childhood acute lymphoblastic leukemia
Abstract
Pediatric acute lymphoblastic leukemia (ALL) is a heterogeneous disease consisting of distinct clinical and biological subtypes that are characterized by specific chromosomal abnormalities or gene mutations. Mutation of genes encoding tyrosine kinases is uncommon in ALL, with the exception of Philadelphia chromosome-positive ALL, where the t(9,22)(q34;q11) translocation encodes the constitutively active BCR-ABL1 tyrosine kinase. We recently identified a poor prognostic subgroup of pediatric BCR-ABL1-negative ALL patients characterized by deletion of IKZF1 (encoding the lymphoid transcription factor IKAROS) and a gene expression signature similar to BCR-ABL1-positive ALL, raising the possibility of activated tyrosine kinase signaling within this leukemia subtype. Here, we report activating mutations in the Janus kinases JAK1 (n = 3), JAK2 (n = 16), and JAK3 (n = 1) in 20 (10.7%) of 187 BCR-ABL1-negative, high-risk pediatric ALL cases. The JAK1 and JAK2 mutations involved highly conserved residues in the kinase and pseudokinase domains and resulted in constitutive JAK-STAT activation and growth factor independence of Ba/F3-EpoR cells. The presence of JAK mutations was significantly associated with alteration of IKZF1 (70% of all JAK-mutated cases and 87.5% of cases with JAK2 mutations; P = 0.001) and deletion of CDKN2A/B (70% of all JAK-mutated cases and 68.9% of JAK2-mutated cases). The JAK-mutated cases had a gene expression signature similar to BCR-ABL1 pediatric ALL, and they had a poor outcome. These results suggest that inhibition of JAK signaling is a logical target for therapeutic intervention in JAK mutated ALL.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

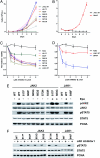
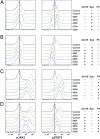
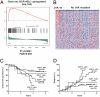
Similar articles
-
Distinct Acute Lymphoblastic Leukemia (ALL)-associated Janus Kinase 3 (JAK3) Mutants Exhibit Different Cytokine-Receptor Requirements and JAK Inhibitor Specificities.J Biol Chem. 2015 Nov 27;290(48):29022-34. doi: 10.1074/jbc.M115.670224. Epub 2015 Oct 7. J Biol Chem. 2015. PMID: 26446793 Free PMC article.
-
Tyrosine kinase fusion genes in pediatric BCR-ABL1-like acute lymphoblastic leukemia.Oncotarget. 2017 Jan 17;8(3):4618-4628. doi: 10.18632/oncotarget.13492. Oncotarget. 2017. PMID: 27894077 Free PMC article.
-
Tyrosine kinome sequencing of pediatric acute lymphoblastic leukemia: a report from the Children's Oncology Group TARGET Project.Blood. 2013 Jan 17;121(3):485-8. doi: 10.1182/blood-2012-04-422691. Epub 2012 Dec 4. Blood. 2013. PMID: 23212523 Free PMC article.
-
The biology of Philadelphia chromosome-like ALL.Best Pract Res Clin Haematol. 2017 Sep;30(3):212-221. doi: 10.1016/j.beha.2017.07.003. Epub 2017 Jul 6. Best Pract Res Clin Haematol. 2017. PMID: 29050694 Review.
-
Targeting Kinase-activating Genetic Lesions to Improve Therapy of Pediatric Acute Lymphoblastic Leukemia.Curr Med Chem. 2018;25(24):2811-2825. doi: 10.2174/0929867324666170727101932. Curr Med Chem. 2018. PMID: 28748759 Review.
Cited by
-
Alternative TEL-JAK2 fusions associated with T-cell acute lymphoblastic leukemia and atypical chronic myelogenous leukemia dissected in zebrafish.Haematologica. 2012 Dec;97(12):1895-903. doi: 10.3324/haematol.2012.064659. Epub 2012 Jun 24. Haematologica. 2012. PMID: 22733019 Free PMC article.
-
Genetic resistance to JAK2 enzymatic inhibitors is overcome by HSP90 inhibition.J Exp Med. 2012 Feb 13;209(2):259-73. doi: 10.1084/jem.20111694. Epub 2012 Jan 23. J Exp Med. 2012. PMID: 22271575 Free PMC article.
-
Therapeutic advances of targeting receptor tyrosine kinases in cancer.Signal Transduct Target Ther. 2024 Aug 14;9(1):201. doi: 10.1038/s41392-024-01899-w. Signal Transduct Target Ther. 2024. PMID: 39138146 Free PMC article. Review.
-
Type II mode of JAK2 inhibition and destabilization are potential therapeutic approaches against the ruxolitinib resistance driven myeloproliferative neoplasms.Front Oncol. 2024 Jul 18;14:1430833. doi: 10.3389/fonc.2024.1430833. eCollection 2024. Front Oncol. 2024. PMID: 39091915 Free PMC article.
-
Challenges and opportunities in childhood cancer drug development.Nat Rev Cancer. 2012 Nov;12(11):776-82. doi: 10.1038/nrc3370. Epub 2012 Oct 11. Nat Rev Cancer. 2012. PMID: 23051845 Review.
References
-
- Pui CH, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet. 2008;371:1030–1043. - PubMed
-
- Mullighan CG, et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007;446:758–764. - PubMed
-
- Mullighan CG, et al. BCR-ABL1 lymphoblastic leukaemia is characterized by the deletion of Ikaros. Nature. 2008;453:110–114. - PubMed
-
- Nachman JB, et al. Augmented post-induction therapy for children with high-risk acute lymphoblastic leukemia and a slow response to initial therapy. N Engl J Med. 1998;338:1663–1671. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA118100/CA/NCI NIH HHS/United States
- U01 CA114762/CA/NCI NIH HHS/United States
- CA114762/CA/NCI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- U10 CA098413/CA/NCI NIH HHS/United States
- N01-C0-12400/PHS HHS/United States
- CA098543/CA/NCI NIH HHS/United States
- T32 HD044331/HD/NICHD NIH HHS/United States
- U01 CA157937/CA/NCI NIH HHS/United States
- U10 CA098543/CA/NCI NIH HHS/United States
- T32 CA128583/CA/NCI NIH HHS/United States
- U10 CA98543/CA/NCI NIH HHS/United States
- U10 CA98413/CA/NCI NIH HHS/United States
- L40 CA142226/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

