Antiepileptic effects of silk-polymer based adenosine release in kindled rats
- PMID: 19460372
- PMCID: PMC2728789
- DOI: 10.1016/j.expneurol.2009.05.018
Antiepileptic effects of silk-polymer based adenosine release in kindled rats
Abstract
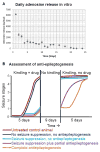
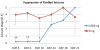
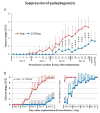
Pharmacotherapy for epilepsy is limited by high incidence of pharmacoresistance and failure to prevent development and progression of epilepsy. Using the rat hippocampal kindling model, we report on the therapeutic potential of novel silk-based polymers engineered to release the anticonvulsant adenosine. Polymers were designed to release 1000 ng adenosine per day during a time span of ten days. In the first experiment rats were kindled by hippocampal electrical stimulation until all animals reacted with stage 5 seizures. Adenosine-releasing or control polymers were then implanted into the infrahippocampal fissure ipsilateral to the site of stimulation. Subsequently, only recipients of adenosine-releasing implants were completely protected from generalized seizures over a period of ten days corresponding to the duration of sustained adenosine release. To monitor seizure development in the presence of adenosine, adenosine-releasing or control polymers were implanted prior to kindling. After 30 stimulations--delivered from days 4 to 8 after implantation--control animals had developed convulsive stage 5 seizures, whereas recipients of adenosine-releasing implants were still protected from convulsive seizures. Kindling was resumed after nine days to allow expiration of adenosine release. During additional 30 stimulations, recipients of adenosine-releasing implants gradually resumed kindling development at seizure stages corresponding to those when kindling was initially suspended, while control rats resumed kindling development at convulsive seizure stages. Blockade of adenosine A1 receptors did not exacerbate seizures in protected animals. We conclude that silk-based adenosine delivery exerts potent anti-ictogenic effects, but might also have at least partial anti-epileptogenic effects. Thus, silk-based adenosine augmentation holds promise for the treatment of epilepsy.
Figures


Comment in
-
Adenosine prevents kindled seizures--an effect as smooth as silk.Epilepsy Curr. 2010 Mar;10(2):51-2. doi: 10.1111/j.1535-7511.2009.01353.x. Epilepsy Curr. 2010. PMID: 20231925 Free PMC article. No abstract available.
Similar articles
-
Silk polymer-based adenosine release: therapeutic potential for epilepsy.Biomaterials. 2008 Sep;29(26):3609-16. doi: 10.1016/j.biomaterials.2008.05.010. Epub 2008 Jun 2. Biomaterials. 2008. PMID: 18514814 Free PMC article.
-
Seizure suppression in kindled rats by intraventricular grafting of an adenosine releasing synthetic polymer.Exp Neurol. 1999 Nov;160(1):164-74. doi: 10.1006/exnr.1999.7209. Exp Neurol. 1999. PMID: 10630201
-
The role of piriform cortex adenosine A1 receptors on hippocampal kindling.Can J Neurol Sci. 2008 May;35(2):226-31. doi: 10.1017/s0317167100008684. Can J Neurol Sci. 2008. PMID: 18574939
-
Pharmacology of glutamate receptor antagonists in the kindling model of epilepsy.Prog Neurobiol. 1998 Apr;54(6):721-41. doi: 10.1016/s0301-0082(97)00092-0. Prog Neurobiol. 1998. PMID: 9560847 Review.
-
Synaptic mechanisms in the kindled epileptic focus: a speculative synthesis.Adv Neurol. 1986;44:411-33. Adv Neurol. 1986. PMID: 2871722 Review.
Cited by
-
Research progress on adenosine in central nervous system diseases.CNS Neurosci Ther. 2019 Sep;25(9):899-910. doi: 10.1111/cns.13190. Epub 2019 Jul 23. CNS Neurosci Ther. 2019. PMID: 31334608 Free PMC article. Review.
-
Bioenergetic Mechanisms of Seizure Control.Front Cell Neurosci. 2018 Oct 8;12:335. doi: 10.3389/fncel.2018.00335. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30349461 Free PMC article. Review.
-
Adenosine-associated delivery systems.J Drug Target. 2015;23(7-8):580-96. doi: 10.3109/1061186X.2015.1058803. J Drug Target. 2015. PMID: 26453156 Free PMC article. Review.
-
Glial adenosine kinase--a neuropathological marker of the epileptic brain.Neurochem Int. 2013 Dec;63(7):688-95. doi: 10.1016/j.neuint.2013.01.028. Epub 2013 Feb 4. Neurochem Int. 2013. PMID: 23385089 Free PMC article. Review.
-
Historical and Current Adenosine Receptor Agonists in Preclinical and Clinical Development.Front Cell Neurosci. 2019 Mar 28;13:124. doi: 10.3389/fncel.2019.00124. eCollection 2019. Front Cell Neurosci. 2019. PMID: 30983976 Free PMC article. Review.
References
-
- Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL. Silk-based biomaterials. Biomaterials. 2003;24:401–416. - PubMed
-
- Anschel DJ, Ortega EL, Kraus AC, Fisher RS. Focally injected adenosine prevents seizures in the rat. Exp Neurol. 2004;190:544–547. - PubMed
-
- Boison D, Huber A, Padrun V, Deglon N, Aebischer P, Mohler H. Seizure suppression by adenosine-releasing cells is independent of seizure frequency. Epilepsia. 2002;43:788–796. - PubMed
-
- Boison D. Adenosine-based cell therapy approaches for pharmacoresistant epilepsies. Neurodegener Dis. 2007a;4:28–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

