Proteasome-caspase-cathepsin sequence leading to tau pathology induced by prostaglandin J2 in neuronal cells
- PMID: 19457109
- PMCID: PMC2889249
- DOI: 10.1111/j.1471-4159.2009.06142.x
Proteasome-caspase-cathepsin sequence leading to tau pathology induced by prostaglandin J2 in neuronal cells
Abstract
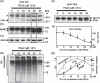
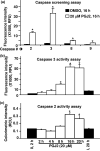
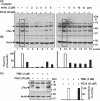
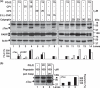
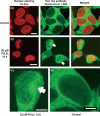
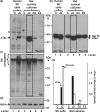
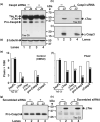
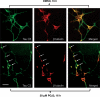
Neurofibrillary tangles (NFT) are a hallmark of Alzheimer's disease. The major neurofibrillary tangle component is tau that is truncated at Asp421 (Deltatau), hyperphosphorylated and aggregates into insoluble paired helical filaments. Alzheimer's disease brains also exhibit signs of inflammation manifested by activated astrocytes and microglia, which produce cytotoxic agents among them prostaglandins. We show that prostaglandin (PG) J2, an endogenous product of inflammation, induces caspase-mediated cleavage of tau, generating Deltatau, an aggregation prone form known to seed tau aggregation prior to neurofibrillary tangle formation. The initial event observed upon PGJ2-treatment of human neuroblastoma SK-N-SH cells was the build-up of ubiquitinated (Ub) proteins indicating an early disruption of the ubiquitin-proteasome pathway. Apoptosis kicked in later, manifested by caspase activation and caspase-mediated cleavage of tau at Asp421 and poly (ADP-ribose) polymerase. Furthermore, cathepsin inhibition stabilized Deltatau suggesting its lysosomal clearance. Upon PGJ2-treatment tau accumulated in a large perinuclear aggregate. In rat E18 cortical neuronal cultures PGJ2-treatment also generated Deltatau detected in dystrophic neurites. Levels of Deltatau were diminished by caspase 3 knockdown using siRNA. PGD2, the precursor of PGJ2, produced some Deltatau. PGE2 generated none. Our data suggest a potential sequence of events triggered by the neurotoxic product of inflammation PGJ2 leading to tau pathology. The accumulation of Ub proteins is an early response. If cells fail to overcome the toxic effects induced by PGJ2, including accumulation of Ub proteins, apoptosis kicks in triggering caspase activation and tau cleavage, the clearance of which by cathepsins could be compromised culminating in tau pathology. Our studies are the first to provide a mechanistic link between inflammation and tau pathology.
Figures


Similar articles
-
Coordination between proteasome impairment and caspase activation leading to TAU pathology: neuroprotection by cAMP.Cell Death Dis. 2012 Jun 21;3(6):e326. doi: 10.1038/cddis.2012.70. Cell Death Dis. 2012. PMID: 22717581 Free PMC article.
-
The role of caspase cleavage of tau in Alzheimer disease neuropathology.J Neuropathol Exp Neurol. 2005 Feb;64(2):104-12. doi: 10.1093/jnen/64.2.104. J Neuropathol Exp Neurol. 2005. PMID: 15751224 Review.
-
Neurotoxic mechanisms by which the USP14 inhibitor IU1 depletes ubiquitinated proteins and Tau in rat cerebral cortical neurons: Relevance to Alzheimer's disease.Biochim Biophys Acta Mol Basis Dis. 2017 Jun;1863(6):1157-1170. doi: 10.1016/j.bbadis.2017.03.017. Epub 2017 Apr 1. Biochim Biophys Acta Mol Basis Dis. 2017. PMID: 28372990 Free PMC article.
-
Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology.J Clin Invest. 2004 Jul;114(1):121-30. doi: 10.1172/JCI20640. J Clin Invest. 2004. PMID: 15232619 Free PMC article.
-
It may take inflammation, phosphorylation and ubiquitination to 'tangle' in Alzheimer's disease.Neurodegener Dis. 2006;3(6):313-9. doi: 10.1159/000095638. Epub 2006 Sep 4. Neurodegener Dis. 2006. PMID: 16954650 Review.
Cited by
-
Ubiquitin/proteasome pathway impairment in neurodegeneration: therapeutic implications.Apoptosis. 2010 Nov;15(11):1292-311. doi: 10.1007/s10495-010-0466-z. Apoptosis. 2010. PMID: 20131003 Free PMC article. Review.
-
Neuroinflammation is not a Prerequisite for Diabetes-induced Tau Phosphorylation.Front Neurosci. 2015 Nov 9;9:432. doi: 10.3389/fnins.2015.00432. eCollection 2015. Front Neurosci. 2015. PMID: 26617484 Free PMC article.
-
Physiological and Pathological Roles of 15-Deoxy-Δ12,14-Prostaglandin J2 in the Central Nervous System and Neurological Diseases.Mol Neurobiol. 2018 Mar;55(3):2227-2248. doi: 10.1007/s12035-017-0435-4. Epub 2017 Mar 16. Mol Neurobiol. 2018. PMID: 28299574 Review.
-
Modulation of Cytosolic Phospholipase A2 as a Potential Therapeutic Strategy for Alzheimer's Disease.J Alzheimers Dis Rep. 2023 Dec 29;7(1):1395-1426. doi: 10.3233/ADR-230075. eCollection 2023. J Alzheimers Dis Rep. 2023. PMID: 38225969 Free PMC article.
-
The Role of Eicosanoids in Alzheimer's Disease.Int J Environ Res Public Health. 2019 Jul 18;16(14):2560. doi: 10.3390/ijerph16142560. Int J Environ Res Public Health. 2019. PMID: 31323750 Free PMC article. Review.
References
-
- Alves-Rodrigues A, Gregori L, Figueiredo-Pereira ME. Ubiquitin, cellular inclusions and their role in neurodegeneration. Trends Neurosci. 1998;21:516–520. - PubMed
-
- Aoun P, Simpkins JW, Agarwal N. Role of PPAR-gamma ligands in neuroprotection against glutamate-induced cytotoxicity in retinal ganglion cells. Invest. Ophthalmol. Vis. Sci. 2003;44:2999–3004. - PubMed
-
- Biedler JL, Roffler-Tarlov S, Schachner M, Freedman LS. Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res. 1978;38:3751–3757. - PubMed
-
- Cho JH, Johnson GV. Glycogen synthase kinase 3 beta induces caspase-cleaved tau aggregation in situ. J. Biol. Chem. 2004;279:54716–54723. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

