Positive transcription elongation factor b activity in compensatory myocardial hypertrophy is regulated by cardiac lineage protein-1
- PMID: 19443839
- PMCID: PMC2774227
- DOI: 10.1161/CIRCRESAHA.108.191726
Positive transcription elongation factor b activity in compensatory myocardial hypertrophy is regulated by cardiac lineage protein-1
Abstract
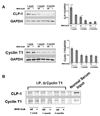
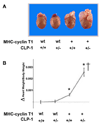
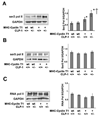
Emerging evidence illustrates the importance of the positive transcription elongation factor (P-TEF)b in control of global RNA synthesis, which constitutes a major feature of the compensatory response to diverse hypertrophic stimuli in cardiomyocytes. P-TEFb complex, composed of cyclin T and cdk9, is critical for elongation of nascent RNA chains via phosphorylation of the carboxyl-terminal domain of RNA polymerase (Pol) II. We and others have shown that the activity of P-TEFb is inhibited by its association with cardiac lineage protein (CLP)-1, the mouse homolog of human HEXIM1, in various physiological and pathological conditions. To investigate the mechanism of control of P-TEFb activity by CLP-1 in cardiac hypertrophy, we used a transgenic mouse model of hypertrophy caused by overexpression of calcineurin in the heart. We observed that the level of CLP-1 associated with P-TEFb was reduced markedly in hypertrophic hearts. We also generated bigenic mice (MHC-cyclin T1/CLP-1(+/-)) by crossing MHC-cyclin T1 transgenic mice with CLP-1 heterozygote. The bigenic mice exhibit enhanced susceptibility to hypertrophy that is accompanied with an increase in cdk9 activity via an increase in serine 2 phosphorylation of carboxyl-terminal domain and an increase in GLUT1/GLUT4 ratio. These mice have compensated systolic function without evidence of fibrosis and reduced lifespan. These data suggest that the reduced level of CLP-1 introduced in the background of elevated levels of cyclin T1 elevates derepression of P-TEFb activity and emphasizes the importance of the role of CLP-1 in the mechanism governing compensatory hypertrophy in cardiomyocytes.
Figures




Comment in
-
Dysregulation of positive transcription elongation factor B and myocardial hypertrophy.Circ Res. 2009 Jun 19;104(12):1327-9. doi: 10.1161/CIRCRESAHA.109.200485. Circ Res. 2009. PMID: 19542017 Free PMC article. No abstract available.
Similar articles
-
Dysregulation of positive transcription elongation factor B and myocardial hypertrophy.Circ Res. 2009 Jun 19;104(12):1327-9. doi: 10.1161/CIRCRESAHA.109.200485. Circ Res. 2009. PMID: 19542017 Free PMC article. No abstract available.
-
Pivotal role of cardiac lineage protein-1 (CLP-1) in transcriptional elongation factor P-TEFb complex formation in cardiac hypertrophy.Cardiovasc Res. 2007 Jul 1;75(1):129-38. doi: 10.1016/j.cardiores.2007.03.019. Epub 2007 Mar 28. Cardiovasc Res. 2007. PMID: 17459355 Free PMC article.
-
The positive transcription elongation factor b is an essential cofactor for the activation of transcription by myocyte enhancer factor 2.J Mol Biol. 2008 Oct 3;382(2):275-87. doi: 10.1016/j.jmb.2008.07.017. Epub 2008 Jul 16. J Mol Biol. 2008. PMID: 18662700 Free PMC article.
-
P-TEFb: The master regulator of transcription elongation.Mol Cell. 2023 Feb 2;83(3):393-403. doi: 10.1016/j.molcel.2022.12.006. Epub 2023 Jan 3. Mol Cell. 2023. PMID: 36599353 Free PMC article. Review.
-
Cyclins that don't cycle--cyclin T/cyclin-dependent kinase-9 determines cardiac muscle cell size.Cell Cycle. 2003 Mar-Apr;2(2):99-104. Cell Cycle. 2003. PMID: 12695656 Review.
Cited by
-
Cardiac lineage protein-1 (CLP-1) regulates cardiac remodeling via transcriptional modulation of diverse hypertrophic and fibrotic responses and angiotensin II-transforming growth factor β (TGF-β1) signaling axis.J Biol Chem. 2012 Apr 13;287(16):13084-93. doi: 10.1074/jbc.M111.288944. Epub 2012 Feb 3. J Biol Chem. 2012. PMID: 22308025 Free PMC article.
-
HMBA is a putative HSP70 activator stimulating HEXIM1 expression that is down-regulated by estrogen.J Steroid Biochem Mol Biol. 2017 Apr;168:91-101. doi: 10.1016/j.jsbmb.2017.02.008. Epub 2017 Feb 14. J Steroid Biochem Mol Biol. 2017. PMID: 28213333 Free PMC article.
-
Negative elongation factor controls energy homeostasis in cardiomyocytes.Cell Rep. 2014 Apr 10;7(1):79-85. doi: 10.1016/j.celrep.2014.02.028. Epub 2014 Mar 20. Cell Rep. 2014. PMID: 24656816 Free PMC article.
-
BET-ting on chromatin-based therapeutics for heart failure.J Mol Cell Cardiol. 2014 Sep;74:98-102. doi: 10.1016/j.yjmcc.2014.05.002. Epub 2014 May 14. J Mol Cell Cardiol. 2014. PMID: 24838003 Free PMC article. Review.
-
The Bin3 RNA methyltransferase targets 7SK RNA to control transcription and translation.Wiley Interdiscip Rev RNA. 2012 Sep-Oct;3(5):633-47. doi: 10.1002/wrna.1123. Epub 2012 Jun 27. Wiley Interdiscip Rev RNA. 2012. PMID: 22740346 Free PMC article. Review.
References
-
- Wagner M, Mascareno E, Siddiqui MA. Cardiac hypertrophy: signal transduction, transcriptional adaptation, and altered growth control. Ann N Y Acad Sci. 1999;874:1–10. - PubMed
-
- MacLellan WR, Schneider MD. Genetic dissection of cardiac growth control pathways. Annu Rev Physiol. 2000;62:289–319. - PubMed
-
- Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. - PubMed
-
- van Empel VP, De Windt LJ. Myocyte hypertrophy and apoptosis: a balancing act. Cardiovasc Res. 2004;63:487–499. - PubMed
-
- Olson EN, Schneider MD. Sizing up the heart: development redux in disease. Genes Dev. 2003;17:1937–1956. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

